常规的农药残留检测一般存在前处理操作复杂, 耗时较长, 方法不够灵敏等问题。 根据氨基与茚三酮显色原理和局域表面等离子体共振(LSPR)增强光吸收原理, 利用紫外-可见吸收光谱对水样品中草甘膦含量进行定量分析, 并利用密度泛函理论进一步分析了显色的类似罗曼紫产物的光吸收增强机理。 草甘膦与茚三酮在钼酸钠催化下反应生成类似罗曼紫产物; 该物质在紫外-可见吸收光谱570 nm处有最大吸收峰, 当其吸附在银纳米粒子(Ag NPs)表面上时, 最大吸收峰蓝移至568 nm处, 同时吸收强度显著提高; 本研究中检出限为2.017 4×10-11 mol·L-1, 显著低于文献中约6.5×10-7 mol·L-1的检出限。 Gaussian 09软件计算得出, 类似罗曼紫产物经由茚三酮的C=O基团垂直吸附在Ag NPs表面, 静电势表明茚三酮的C=O基团优先与Ag稳定相互作用并形成Ag—O键, C=O基团和C—N基团构成了π键共轭系统; 连接草甘膦和茚三酮之间的C—N键是类似罗曼紫产物的生色团。 因此, 茚三酮衍生法可用于间接检测水样品中草甘膦, 银纳米粒子LSPR效应增强了光吸收强度, 比常规方法具有更高的灵敏度。
Biography: XU Meng-lei, female, (1988—), a lecturer of State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University e-mail: xumenglei@jlu.edu.cn
Conventional pesticide residue detection still suffers from numerous steps, is long time-consuming, and insufficient delicacy. According to ninhydrin colouring and the principle of localized surface plasmon resonance (LSPR) enhancing absorption, glyphosate in water samples can be detected by ultraviolet-visible spectroscopy (UV-Vis). Furthermore, density functional theory is used to analyze the enhancement mechanism of absorption of purple color dye (PD) products. The PD product displays a maximum absorption of around 570 nm when glyphosate reacts with ninhydrin is detected by UV-Vis. There are slight shifts from 570 nm in the UV-Vis spectrum to 568 nm with a stronger peak when the PD product is absorbed on Ag NPs, and the limit of detection at 2.017 4×10-11 mol·L-1, which is much lower than 6.5×10-7 mol·L-1 limit of detection reported. Gaussian 09 software carried out that the PD product would attach to Ag NPs via an Ag—O bond through ninhydrin's C=O group vertically. MEP mapping provides C=O group interaction with Ag NPs stable, C=O group coupled C—N comprise a large π-conjugated system in their plane. The color of the C—N group is blue, which suggests that C—N coupled with the C=O group are the chromophore in the PD product. Thus, an indirect detection method derived from ninhydrin can be used for glyphosate detection in water samples. The LSPR effect of Ag NPs enhances the absorption intensity with higher sensitivity than the conventional method.
Glyphosate (C3H8NO5P) is the highest volume herbicide used worldwide with a potential toxic pollutant in the environment[1]. The common determination methods use chromatographs coupled to various detectors[2]. These methods are sensitive. However, they still suffered with numerous steps for the purification and derivatization due to their highly polar and no fluorescence group[3]. Therefore, developing a new analytical technique for monitoring glyphosate is necessary[4, 5].
The colorimetric method has been found simple and economical for determining of glyphosate[6]. Glyphosate reacts with ninhydrin from its amino group, and the product is purple, similar to Ruhemann's purple product, while this ‘ Purple Color Dye (PD) Product' is slightly different. This conventional colorimetric method appeared to have a limit of quantification (LOQ) at 0.04 μ g· mL-1 in water samples in UV-Vis[7]. Localized surface plasmon resonance (LSPR) is a collective oscillation of conduction band electrons in metal nanoparticles (NPs) driven by the electromagnetic field of the incident light, which could significantly increase the light absorption and the generation of the photon carriers. Metal NPs have been widely exploited as colorimetric sensors or probes for detecting a variety of analytes because of their high extinction coefficients and strong LSPR properties[8]. In this paper, we describe a simple and cheap screening method for detecting glyphosate in water enhanced by LSPR of Ag NPs based on the ninhydrin reaction aim of high sensitivity and simplicity.
Silver nitrate (AgNO3) was purchased from Sigma-Aldrich Chemical Co., Ltd. Glyphosate, sodium citrate (Na3C6H5O7· 2H2O), sodium molybdate (Na2MoO4) and ninhydrin were obtained from Aladdin Industrial Corporation. Tap water was collected without further clean-up for real sample determination.
UV-Vis extinction spectra were measured with a lambda1050+ spectrophotometer (Perkinelmer).
Ag NPs were prepared as follows, 36.0 mg AgNO3 was added into 200.0 mL H2O first, and using sodium citrate (1% ω /V, 4 mL) as a reducing agent. A grey-green colloid formed and was naturally cooled at room temperature, after heating at 85 ℃ for 40 min.
Ninhydrin working reagent: Nninhydrin solution (5%, ω /V)+water+acetate buffer (0.4 mol· L-1, pH 5.5) (2:1:1, V/V/V). Mixed working solutions: Ninhydrin working reagent+5% Na2MoO4+sample/glyphosate solution (1:1:1, V/V/V) were heated in boiling water for 30 min; and mixed with Ag NPs (1:1, V/V).
Water samples were only clean-up by a 0.45 μ m microporous ultrafiltration membrane, and then added different concentrations of glyphosate.
All geometries were optimized using the B3LYP exchange-correlation function. The 6-311++G(d, p) basis set was used for the H, C, O, N, P atoms, and LanL2DZ level for Ag NPs. All calculations were carried out using the Gaussian 09 software program.
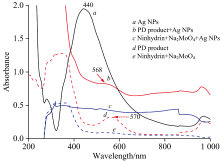
The Ag NPs used in this study have a maximum absorption of around 440 nm (Fig.1a). The PD product displays a maximum absorption of around 570 nm (Fig.1d). When the PD absorbed on Ag NPs, there are slight shifts from 570 nm in the UV-Vis spectrum to 568 nm with a stronger peak (Fig.1b). This should originate from the interaction between the Ag NPs and the PD product molecules.
The optimized geometry of the PD product [Fig.2(a)], its Ag [Fig.2(b)] and Ag3 [Fig.2(c)] complexes were geometry-optimized.
 | Fig.2 Molecular structures and electrostatic potential (MEP) mapping of purple color dye (PD) product and its Ag complexes (a): PD product; (b): Ag-PD product; (c): Ag3-PD product; (d): MEP mapping |
The PD product includes two moieties, a ninhydrin molecule and a glyphosate molecule, connected by a C— N group [Fig.2(a)]. All the atoms in the ninhydrin part lie in the same plane and include the N16 atom with a large π -conjugated system. Thus, the PD product displays a maximum absorption peak at 570 nm due to this chromophore and auxochrome. Negative charges (electrophilic regions) are represented in red, while positive charges (nucleophilic regions) are represented in green; the MEP increases in the order of red< orange< yellow< green< blue [Fig.2(d)]. The electron density of the ninhydrin C=O group is higher than that of the other atoms, the color is green. These results suggested that this atom preferentially interacts with Ag and, thus, that the PD product would attach to Ag via an Ag— O bond. Significantly, the color of the C— N group is blue, which suggests that C— N coupled with the C=O group are the chromophore in the PD product.
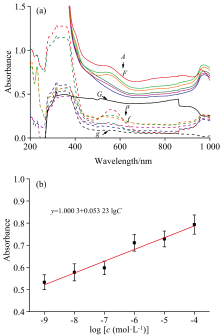
Representative concentration-dependent UV-Vis spectra of the PD products were shown in Fig.3(a). The absorbance intensity at 568 nm shows a good linear relationship with the concentrations of PD product in the range of 1.00× 10-9~1.00× 10-4 mol· L-1 (y=1.000 3+0.053 23× lgc, R2=0.939 5) [Fig.3(b)]. The LOD is thus calculated to be 2.017 4× 10-11 mol· L-1.
Recovery experiments were performed in each batch analysis to validate the method by spiking samples in five replicates at 1.00× 10-8, 1.00× 10-7, 1.00× 10-6 and 1.00× 10-5 mol· L-1. The recoveries of water samples ranged from 71.26%± 1.63% to 114.68%± 11.30% without further clean-up. After further clean-up process, the recoveries ranged from 88.76%± 4.81% to 98.51%± 4.16%. It should be some unknown molecules in tap water that interfered with the adsorption of the target molecules on Ag NPs.
| Table 1 Recoveries of glyphosate spiked into tap water |
Influence of common cations and anions, such as Na+, K+, Mg2+, Ca2+, Cl-, S
| Table 2 Influence of coexisting substances on glyphosate detection in water samples |
Ninhydrin, glyphosate and Na2MoO4 are colorless and transparent solutions, while the C=O double bond of ninhydrin as chromophore linked with N— H bond from glyphosate as auxochrome, thus PD product displays a maximum absorption peak at 570 nm. The LSPR phenomenon of Ag NPs significantly increases the light absorption and the generation of the photon carriers. Thus, the absorbance of PD product absorbed on Ag NPs is stronger than PD product only. When glyphosate concentration is lower than 1.0× 10-7 mol· L-1, spectra of PD product absorbed on Ag NPs are still different from the blank. C— N coupled with the C=O group are the chromophore in the PD product, and the PD product would attach to Ag via an Ag— O bond from the ninhydrin part. While only ninhydrin, or mixed with catalyzer Na2MoO4, can not be absorbed on Ag NPs. Thus, spectra of PD products absorbed by Ag NPs provided content information on glyphosate. LSPR phenomenon of Ag NPs improved sensitivity in glyphosate detection based on the ninhydrin reaction.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|