e-mail: lamiaalbedair@gmail.com
2,4-Dichlorophenoxyacetic acid (2,4-D) is a board-leaf selective herbicide and globally used in agricultural activities. Complexation mode, spectroscopic investigations and biological properties of complexes formed between 2,4-D (C6H3Cl2OCH2·COOH; HL) with Zn(Ⅱ), Cu(Ⅱ), Ni(Ⅱ), Co(Ⅱ), and Mn(Ⅱ) metal ions were investigated. To characterize the binding mode between 2,4-D and the metal ions, many physicochemical approaches were employed. The complexes obtained are characterized quantitatively and qualitatively by using micro elemental analysis, FTIR spectroscopy, UV-Vis spectroscopy,1H-NMR, and magnetic susceptibility measurements. Results of these approaches suggested that the gross formula of the complexes obtained with the metal ions were [ZnL2](2H2O (1), [CuL2(H2O)2] (2), [NiL2](3H2O (3), [CoL2(H2O)2] (4), and [MnL2(H2O)2] (5). In all complexes, two L- anion were coordinated the metal ion by their bidentate carboxylate groups. From the spectral study, all the complexes obtained as monomeric structure and the metals center moieties are six-coordinated with octahedral geometry except Ni(Ⅱ) and Zn(Ⅱ) complexes which existed as a tetrahedral and square pyramidal geometry respectively. The complexes were screened in vitro against several microbes (fungi and bacteria) using Kirby-Bauer disc diffusion method, and data has demonstrated that complex 3 showed excellent antifungal activity.
The uses of herbicides have greatly expanded and increased widely in agricultural fields[1, 2, 3, 4]. Four common chlorophenoxyacetic acids (PAAs) are consider as important herbicides and have been extensively used in agriculture there are 2, 4-dichlorophenoxyacetic acid (2, 4-D), 2-(2, 4-dichlorophenoxy)propionic acid (2, 4-DP), 4-chlorophenoxyacetic acid (4-CPA), and dicamba (DICA)[5, 6, 7, 8]. The 2, 4-D is an aromatic acid appeared as a white to yellow solid powder with molecular formula Cl2C6H3OCH2· COOH, molecular weight 221.04 g· mol-1, and melting point 140.5 ℃, slightly volatile and a polar molecule. It belongs to PAAs herbicides that are potentially toxic and carcinogenic to human, easily accumulated in the body and cause great harm[9, 10, 11, 12]. The widely frequently and extensively usage of 2, 4-D by farmers in agricultural fields to prevent the growth and kill most broadleaf weeds[13, 14, 15, 16]. Since the 1940, 2, 4-D has been approved for use as a selective herbicide. It was one of the ingredients of a powerful herbicide used during the Vietnam war named “ Agent Orange” to eliminate crops and forest cover[17]. It applies through spraying onto the leaves of crops to control the development of broadleaves weeds in a wider range of crops like soybeans, wheat, hay, and corn. It also widely used in ball fields, parks, forests, golf courses, and lawns[13, 18]. 2, 4-D herbicide causes uncontrolled growth and eventual kills the unwanted grassy weeds by mimicking the plant growth regulator leading to disorganized plant growth and death[19]. The widespread, extended and continuous use of 2, 4-D resulted in 2, 4-D and its transformation products (phenol, 2, 4-dichlorophenol, 4-chlorophenol, and 2-chlorophenol) contaminated natural water, soil resources, and agricultural food products[20, 21]. The presence of 2, 4-D in environment and foods is considered as an environmental problem of major concern and a potential risk for human health and ecosystems. Also, there is concern regarding the risks of human exposure to 2, 4-D occurring directly during loading, mixing, manufacturing, and applying processes of the 2, 4-D[22, 23, 24, 25, 26]. Since 2015, 2, 4-D was suspected to be carcinogenic and banned in several countries[17]. Different strategic processes have been used for removal of 2, 4-D from environments such as dechlorination, photocatalysis, oxidation, and adsorption methods[27]. But not any of these strategies based on metal complexation yet.
Knowledge about the interaction of 2, 4-D with metal ions is important in order to improve an strategic process for its removal from environments based on metal complexation. Several works reported on this interaction. N. Naz et al, [28] prepared and characterized five new tri- and di-organotin (Ⅵ ) derivatives of 2, 4-D having the general formula: Oct2SnL2, Bu2SnL2, Me2SnL2, Bu3SnL, and Me3SnL (L: Cl2C6H3OCH2· COO-). A. Drzewiecka-Antonik et al, [29] investigated the interaction between 2, 4-D with the metal ions Cu(Ⅱ ), Ni(Ⅱ ), and Co(Ⅱ ) in aqueous media experimentally and theoretically using the XAFS and DFT analyses. Their findings indicated mononuclear complexes with nearly centrosymmetric arrangements of O atom around the metal ion. J. Kobył ecka et al, [30] conducted a thermal study (TG, DTG, and DTA) on the interaction between 2, 4-D and the metal ions Mg(Ⅱ ), Ca(Ⅱ ), Fe(Ⅱ ), and Hg(Ⅱ ). They indicated that the obtained complexes have the general formula ML2(nH2O, and the complexes obtained with Mg(Ⅱ ) and Ca(Ⅱ ) ions are highly thermal stabile than that obtained with Fe(Ⅱ ) and Hg(Ⅱ ) ions. Due to the versatile coordination modes of 2, 4-D with metal ions, it was used in the synthesis of metal-organic frameworks (MOF) of polymeric or mononuclear monomeric complexes, several of these frameworks were collected as single crystals contain 2, 4-D in mixed-ligand complex[31, 32, 33, 34, 35, 36]. This work was done to (1) prepare five complexes of 2, 4-D as ligand (HL) with the metal ions Zn(Ⅱ ), Cu(Ⅱ ), Ni(Ⅱ ), Co(Ⅱ ), and Mn(Ⅱ ) under the following conditions: solvent; MeOH∶ H2O (1∶ 1), Media; neutral (pH 7), reaction temperature; 60 ℃, and molar ratio; 2∶ 1 (Ligand∶ Metal), (2) characterize the resulting complexes with elemental analysis, magnetic moment, IR, 1H NMR, and UV(Vis techniques, and (3) their biological activities towards several fungal and bacterial microbial strains.
All starting materials used in the experiments were of reagent chemical grade and used as received without further purification. The solvents used in preparation and physical measurements were of analytical reagent grade. Water used in preparations is Milli-Q purified water (Milli-Q system, Millipore, Bedford, MA, USA). 2, 4-D (Cl2C6H3OCH2CO2H; 221.04 g· mol-1; purity 97%), zinc chloride (ZnCl2; 136.30 g· mol-1; purity ≥ 99.99%), copper(Ⅱ ) chloride dihydrate (CuCl2· 2H2O; 170.48 g· mol-1; purity ≥ 99.95%), nickel(Ⅱ ) chloride hexahydrate (NiCl2· 6H2O; 237.69 g· mol-1; purity 99.9%), cobalt(Ⅱ ) chloride hexahydrate (CoCl2· 6H2O; 237.93 g· mol-1; purity 98.0%), and manganese(Ⅱ ) chloride tetrahydrate (MnCl2· 4H2O; 197.91 g· mol-1; purity ≥ 98.0%) were bought from Sigma-Aldrich Co., (St Louis, MO, USA).
Elemental analyzer (model PE 2400 CHN) was performed to conduct the microanalyses (C% and H%) for the synthesized complexes. The IR spectra were obtained by using an infrared spectrometer (model Bruker FT-IR) in the region 400~4 000 cm-1. The 1H NMR spectra were obtained by using a NMR spectrometer (model Bruker DRX-250; 600 MHz; DMSO-d6 solvent). Electronic spectra were obtained by using an UV-Vis spectrometer (model UV2-Unicam; DMSO solvent) in the region 200~800 nm.
The complexes were synthesized according to the following procedure: An aqueous solution containing 1 mmol (20 mL) of a metal chloride (ZnCl2, CuCl2· H2O, NiCl2· 6H2O, CoCl2· 6H2O, or MnCl2· 4H2O) was added to a MeOH solution containing 2 mol (20 mL) of 2, 4-D under continuous stirring. A few drops of conc. ammonium (NH3) were added till the pH of the mixture reached 7. At this point a colored precipitate begins to formed. The mixture was stirred for 20 minutes at 60 ℃, then cooled at room temperature to ensure the completeness of the precipitation and filtered. All the complexes with the Zn(Ⅱ ), Cu(Ⅱ ), Ni(Ⅱ ), Co(Ⅱ ), and Mn(Ⅱ ) ions were prepared by the same procedure, and the color of the resulting precipitate was yellowish white, greenish blue, oily green, crimson red, brown, respectively. These colored precipitates were thoroughly washed and dried in an oven at 70 ℃.
The modified Bauer-Kirby disc diffusion method[37, 38, 39] was applied to assay the antifungal and antibacterial properties of the synthesized complexes towards to fungi organisms C. albicans and A. flavus and two Gram-positive strains (B. subtilis and S. aureus) and two Gram-negative strains (P. aeruginosa and E. coli.).
The HL ligand was dissolved in methanol solvent, where the Zn(Ⅱ ), Cu(Ⅱ ), Ni(Ⅱ ), Co(Ⅱ ), and Mn(Ⅱ ) chlorides were dissolved in Milli-Q purified water. The reaction between the ligand and each metal ion was carried out under four conditions; ① solvent was MeOH∶ H2O (1∶ 1), ② media was neutral (pH 7), ③ reaction temperature was 60 ℃, and ④ molar reaction was 2∶ 1 (ligand to metal). Under these conditions, the obtained complexes with Zn(Ⅱ ), Cu(Ⅱ ), Ni(Ⅱ ), Co(Ⅱ ), and Mn(Ⅱ ) ions have the gross formula of C16H14Cl4O8Zn (541.46), C16H14Cl4O8Cu (539.63), C16H16Cl4O9Ni(552.77), C16H14Cl4O8Co (535), and C16H14Cl4O8Mn (531), respectively, based on the elemental analysis date listed in Table 1. These data suggest that the obtained complexes are formulated as [ZnL2](2H2O (1), [CuL2(H2O)2] (2), [NiL2](3H2O (3), [CoL2(H2O)2] (4), and [MnL2(H2O)2] (5), respectively (L-: Cl2C6H3OCH2· COO).
| Table 1 Elemental analysis data of 1, 2, 3, 4, and 5 complexes |
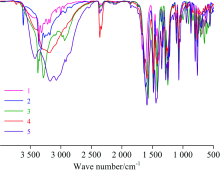
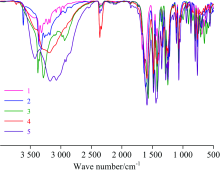
Figure 1 shows the measured IR spectra for complexes 1, 2, 3, 4, and 5, where Table 2 lists the main vibrational assignments obtained from these spectra. Here we discussed the main vibrational assignments that characterized the ligand[40] and the obtained complexes: The IR spectrum of HL was characterized by a broad band centered at 3 445 cm-1 assigned to the carboxylic acid O— H stretching mode. The O— H in-plane plane bending vibration is vibrated at 1 393 cm-1, where the out-of-plane bending vibration is vibrated at 796 cm-1. In the obtained complexes, the band of 3 445 cm-1 is absent, instead, broad absorption bands ranged from 3 400 to 3 260 cm-1 are observed in the IR spectra of the obtained complexes. These bands were characterized to the OH group ν (O— H) of water suggesting the existence of latticed or coordinated water molecules. The IR spectrum of HL was characterized by a very intense absorption band at 1 733 cm-1 due to the stretching vibrations of carboxyl C& #61; O of — COOH group, which was no longer found in the IR spectra of the obtained complexes. After the deprotonation and complexation of — COOH group of HL, two new bands were appeared for the obtained complexes at around 1 479~1 445 cm-1 due to the ν sym(COO-) vibrations, and at around 1 610~1 571 cm-1 due to the ν asym(COO-) vibrations of the carboxylate group. The existing of the characteristic carboxylate bands suggests that ligand-metal binding occurs only through oxygen atoms in the carboxylate group while not through the oxygen atom of ether group or the Cl atoms. According to the Nakamoto criterion, the carboxylate group (COO-) can adopt various coordination modes based oo the differences between the ν as(COO-) and ν s(COO-) values [Δ ν =ν as-ν s] of the complex compared to the free ligand anion[41]. The band frequency differences (( for the complexes are in the range of (Δ ν =165~118), which are lower than that of the ligand sodium salt (NaL), suggests a bidentate corrdination mode for the carboxylate group (COO-) in all the obtained complexes. In the IR spectra of the obtained complexes, the stretching vibrations of Zn— O, Cu— O, Ni— O, Co— O, and Mn— O bond were observed as a weak bands at 555, 552, 580, 550, and 567 cm-1, respectively[42].
| Table 2 FT-IR data (cm-1) for HL ligand and complexes 1, 2, 3, 4, and 5 |
The 1H NMR spectrum of complex 1 was determined in DMSO-d6 solvent at room temperature and compared with the free ligand. The 1H NMR chemical shift for the HL molecule is: δ =4.36 (s, 2H, CH2), 6.65 (d, J=9.00, 1H, C6H phenyl group), 7.04 (d, J=2.4, 1H, C5H phenyl group), 7.23 (s, 1H, C3H phenyl group), 12.55 (s, 1H, COOH). The chemical shift for complex 1 is: δ =4.57 (s, 4H, 2CH2), 6.85 (d, J=9.00, 2H, C6H phenyl group), 7.14 (d, J=2.4, 2H, C5H phenyl group), 7.33 (s, 2H, C3H phenyl group). The HL molecule produced six signals in its 1H NMR spectrum, and all of these proton resonances were found in the spectrum of the complex 1, except that of the COOH group which was no longer observed. In the spectrum of the free ligand, the aromatic protons were resonated in the 6.65~7.23 ppm range, the methylene protons were observed at 4.36 ppm, and the proton of the COOH group was observed at 12.55 ppm. In the spectrum of complex 1, all the aromatic protons from carbons numbered C3, C5, and C6 were represented down-field shifts. The methylene protons were undergone down-field shifted, and exhibited a definite singlet at 4.57 ppm. The protons of methylene group and aromatic protons from carbons numbered C6 which are close to the COO group showed the strongest down-field shift because of ligand to metal charge transfer which further confirmed the complex formation[28].
The UV-Vis. spectra of complexes 1, 2, 3, 4, and 5 were recorded in DMSO solvent over the wavelength range from 200 nm to 800 nm at room temperature. The absorption spectrum of HL exhibits three bands at 228, 282 and 291 nm corresponded to the groups C— Cl, benzene C& #61; C, and C— O or C& #61; O in its molecular structure[43]. After HL complexed with the metal ions, a very strong broad band was appeared with two maxiums at 274 nm and at 296~300 nm. The first maxium was corresponding to the π → π * transitions, where the second one was corresponding to the n→ π * transitions. The weak broad band at 660, 650, and 525 nm found in the spectrum of complex 2, 3, and 4, respectively, may be assigned to the ligand-to-metal charge transfer bands (LMCTs)[44, 45]. The μ eff values for complexes 2, 3, 4, and 5 were 1.9, 3.8, 4.8, and 4.5 B.M., respectively. These values suggest a paramagnetic property for complexes 2, 4, and 5 with an octahedral structure[46, 47, 48]. Complex 4’ s higher μ eff value suggests that it is likely a high spin complex[49]. At room temperature, the μ eff value for tetrahedral complexes is > 3.3 B.M., usually ranged from 3.4 to 3.9 B.M. The μ eff value of complex containing Ni(Ⅱ ) ions falls within in this range (3.8 B.M.), indicating that complex 3 has a tetrahedral stereochemistry. Complex containing Zn(Ⅱ ) ions is diamagnetic, as expected for d10 configuration. Structures of complexes 1, 2, 3, 4, and 5 were proposed based on the spectral, magnetic, and elemental results and presented in Figure 2.
All the obtained complexes as well as the free ligand were screened in vitro for antifungal properties towards to fungi organisms C. albicans and A. flavus., and for antibacterial properties towards to two Gram-positive organisms (B. subtilis and S. aureus) and two Gram-negative organisms (P. aeruginosa and E. coli.). The antibiotic drug Amphotericin B was used to compare the antifungal results of free ligand and the obtained complexes. Zones of inhibition (in mm· mg-1) observed for this antibiotic were 19 and 18 mm· mg-1 against C. albicans and A. flavus, respectively. The antibiotic drug Tetracycline was used to compare the antibacterial results of free ligand and the obtained complexes. Zones of inhibition (in mm· mg-1) observed for this antibiotic were 34, 30, 34, and 32 mm· mg-1 against B. subtilis, S. aureus, P. aeruginosa, and E. coli, respectively. The results showed that all of the complexes as well as the free ligand were found inactive against A. flavus microbe, except complex 3, which show strong activity against this microbe, with a zone of inhibition of 16 mm· mg-1, which was equal to 89% of the activity of the antifungal drug (Amphotericin B). Interestingly, complex 3 is the only complex that exhibited excellent activity against C. albicans microbe, showing maximum zone of inhibition of 20 mm· mg-1, which was higher activity than that of the antifungal drug (Amphotericin B). Complexes 2, 4, and 5 showed moderate activity against this microbe with zone of inhibition in the range 10~12 mm· mg-1. All of the complexes were found active against all the tested bacterial strains, but with moderate level of lethality, with zone of inhibition in the range 12~19 mm· mg-1. Only complex 1 and 4 and exhibited good activity against P. aeruginosa microbe, with a zone of inhibition of 21 and 22 mm· mg-1, respectively. Complexes 1 and 4 were the most potent complexes showing activity against all the tested bacterial strains compared with the other complexes. It’ s important to note that the synthesized complexes are more potent than the free ligand against all the tested microbes.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|