e-mail: drsamaraljazzar2020@gmail.com
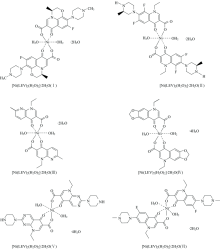
Quinolone has a broad spectrum of synthetic antibiotics with a strong therapeutic effect and quinolone is a term used for chemical treatments used to treat a powerful bacteria. Quinolones are divided into 4 generations according to the bacterial spectrum, the majority of quinolones used clinically belong to the sub fluoroquinolones group, which has a fluorine atom linked to the central ring system, usually on its carbon atom 6 or 7. Herein in this article, six new nickel(Ⅱ) complexes (Ⅰ—Ⅵ) have been synthesized in aqueous alkaline media at pH ranged 8-9, the chemical reactions take place between levofloxacin (HLEV), lomefloxacin (HLOM), nalidixic acid (HNLA), oxolonic acid (HOXO), pipemidic acid (HPIP), and pefloxacin mesylate (HPEF) with nickel(Ⅱ) nitrate hexahydrate. The microanalytical (percentage of carbon, hydrogen and nitrogen), molar conductance ( Λm), Infrared (FTIR) spectra, electronic (UV-Vis) spectra, and effective magnetic moment instrumentals were used to identify the suggested structures and their surface morphology. According the analytical and spectroscopic analyses, the stoichiometry between nickel(Ⅱ) metal ion and drug ligands was found to be 1∶2 with general formula as [Ni(L)2(H2O)2]· xH2O (L=LEV (Ⅰ), LOM (Ⅱ), NAL (Ⅲ), OXO (Ⅳ), PIP (V), and PEF (Ⅵ); x=2 or 4). By the comparison between FTIR spectra of quinolone drugs and their complexes, it can be deduced that all the drug ligands act as a bidentate chelates through oxygen atoms of pyridine ring and carboxylate group. The electronic configuration of all synthesized nickel(Ⅱ) complexes were octahedral geometry which confirmed based on the values of magnetic susceptibility and the electronic transition bands.
Quinolones are an important antibacterial drug family, because of their wide medicinal applications[1] against both Gram-positive and Gram-negative bacteria. The essential structure of quinolone drug consists of bicyclic ring fitted with five essential substituted places at N-1, carboxylic group at C-3, carbonyl group at C-4, fluorine atom at C-6 and piperazinyl or methyl piperazinyl groups at C-7. The presence of a different functional group at sites C-7 or N-1 affects on microbiological and pharmacokinetic properties[2]. The quinolone drugs have a good ability to form complexity towards different metal ions that act as a unidentate, bidentate and bridging bidentate chelate[3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26]. Quinolone and fluoroquinolones drugs are binding as a bidentate fashion via oxygen atoms of carboxylic group and pyridin-4-one ring, uni-dentate chelate via nitrogen atom of piperazinyl ring, and in some rare cases the quinolones drugs are coordinated as a bidentate toward metal ions through the two oxygen atoms of carboxylic group or through two nitrogen atoms of piperazinyl ring[3]. There are number of quinolone (HNAL, HOXO and HPIP) and fluoroquinolones (HLEV, HLOM and HPEF) metal complexes have been synthesized and characterized in literature survey in order to recognized the speculated structures[3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28]. These can be summarized as follows regarding the six quinolone drugs which were studied in this article (Table 1).
| Table 1 Literature survey of HLEV, HLOM, HNAL, HOXO, HPIP, and HPEF metal complexes |
This article aimed to synthesis and spectroscopic characterizations for the six new complexes associated from the chemical interaction between HLEV, HLOM, HNAL, HOXO, HPIP, and HPEF drug chelates with Ni(NO3)2· 6H2O by 1∶ 2 (M∶ L) ratio in alkaline media. The chelation behavior and structural elucidation of the nickel(Ⅱ ) complexes have been built up according to elemental analysis, molar conductance, magnetic, FTIR, and electronic UV(Vis spectroscopic analyses.
The chemical reagents (Ni(NO3)2· 6H2O, dimethylsulfoxide, levofloxacin, lomefloxacin, nalidixic acid, oxolonic acid, pipemidic acid, and pefloxacin mesylate) received in this study were of analytical grade and used without further purifications. These chemicals were purchased from Aldrich-Sigma chemical company.
The microanalytical and spectroscopic analysis that used to discussed the chemical formula of nickel(Ⅱ ) quinolone complexes can be summarized in Table 2.
| Table 2 The type of analysis and the model of instrument |
The six solid nickel(Ⅱ ) quinolone complexes were synthesized by mixing 2.0 mmol HLEV, HLOM, HNAL, HOXO, HPIP or HPEF drugs in 50 mL of methanol solvent with 1.0 mmol of Ni(NO3)2· 6H2O in 20 mL of distilled water. The reaction mixtures were adjust the pH value between 8~9 using ammonia solution (5%), then the mixtures were refluxed on the hotplate for three hours till the precipitate appeared. After cooling to room temperature, the green solid complexes were filtered off and washed with a hot methanol and little amount of distilled water in order to remove the un-reacted chemicals. The clean precipitates were dried and stored in a glass desiccators over anhydrous CaCl2. The yield of the isolated products was ranged between 77%~74%. The melting points of synthesized nickel(Ⅱ ) complexes have a higher m.p over > 250 ℃.
The green solid nickel(Ⅱ ) quinolone complexes were well characterized using elemental analysis, magnetic measurements, molar conductance, FTIR, and electronic spectroscopy. Because of these complexes have an amorphous status, so the X-ray crystal structure didn’ t performed.
The elemental analysis (calculated/Found) and physical values of the six new nickel(Ⅱ ) quinolone complexes are listed in Table 3. The experimental percentage (%) of each elements C, H, N and nickel metal in situ synthesized complexes were matched with the theoretical values, which confirmed the speculated structures of the six nickel(Ⅱ ) complexes (Fig.1). The synthesized Ni(Ⅱ ) complexes were stable in air and insoluble in most organic solvent but soluble in dimethylsulfoxide (DMSO) and dimethylformamide (DMF) with warming. The molar conductivities (Λ m) values are located within 9~12 ohm-1· cm2· mol-1, which were considered as non-electrolytes nature[29].
| Table 3 Microanalytical and physical data of Ni(Ⅱ ) quinolone complexes |
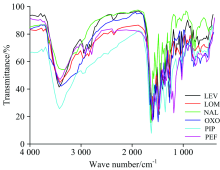
The infrared spectra of free quinolone drugs (HLEV, HLOM, HNAL, HOXO, HPIP, and HPEF) and corresponding nickel(Ⅱ ) complexes (Fig.2) have been assigned to identify the site of coordination and speculated structures (Table 4). Regarding the six Ni(Ⅱ ) complexes, the stretching vibration bands of ν (C& #61; O)COOH carboxylic group which are existed at 1 708~1 728 cm-1 in the spectra of the free HLEV, HLOM, HNAL, HOXO, HPIP, and HPEF ligands are disappeared as well as the frequencies take place at 1 618~1 639 cm-1 due to ν (C& #61; O)pyridone are shifted to lower wavenumbers after complexation. These results confirm that nickel(Ⅱ ) metal ions is coordinated to chelate molecule through oxygen atoms of deprotonated carboxylic group and oxygen of pyridine ring. Table 4 includes the frequencies data of the vibrations of carboxylate anion for the nickel(Ⅱ ) complexes as well as differences in the values of these vibrations Δ (COO). According to Nakamoto, this allows the assessment of the metal-acid coordination mode[30]. In the case of LEV (Ⅰ ), LOM (Ⅱ ), NAL (Ⅲ ), OXO (Ⅳ ), PIP (Ⅴ ), and PEF (Ⅵ ) nickel(Ⅱ ) complexes, the difference in wavenumbers of stretching vibrations of asymmetric ν as(COO) and asymmetric ν s(COO) carboxylate anions (Δ ν =ν asCOO-ν sCOO) for all nickel(Ⅱ ) complexes is higher than for sodium salts (304~328 cm-1) indicates a mono-dentate chelate of metal-to-ligand coordination by a carboxyl group. The broad band at 3 433 cm-1 is attributed to the stretching vibrations of coordinated and uncoordinated water molecules. The three distinguish motions of water molecules, δ r(H2O), δ w(H2O), and δ t(H2O) are observed at 766, 550, 710 cm-1, respectively with agreement with aquo-complexes[30]. The presence of new stretching vibration bands within 500~400 cm-1 range are assigned to the frequencies of ν (Ni— O) vibrations in case of nickel(Ⅱ ) complexes (Ⅰ — Ⅵ )[30].
| Table 4 FTIR assignments of Ni(Ⅱ ) quinolone complexes (Ⅰ — Ⅵ ) |
The electronic spectra of six nickel(Ⅱ ) quinolone complexes contains three absortion bands exhibited at three regions as 13 333~14 388, 14 535~15 674, and 24 390~25 707 cm-1, these electronic transition bands are assigned to 3A2g→ 3T2g (F), (ν 1); 3A2g→ 3T1g (F), (ν 2) and 3A2g→ 3T2g (P), (ν 3) transitions, respectively. The divide data of ν 2/ν 1 ratios concerning the synthesized nickel(Ⅱ ) complexes are presence at 1.04~1.16, this results located within the range of an octahedral geometry for the Ni(Ⅱ ) complexes (Table 5)[31]. The low values of ν 2/ν 1 are due to the strong interaction between 3T1g (P) and 3T1g (F) states. The Racah inter-electronic repulsion (B), ligand field splitting energy (10 Dq), covalency factor (β ) and ligand field stabilization energy (LFSE) as a physical spectroscopic parameters have been estimated according to the electronic spectra. The B parameter is low in comparison with free nickel(Ⅱ ) ion (1 038 cm-1), this meaning that nickel(Ⅱ ) complexes have a covalent character. The covalency factor (β ) of the six nickel(Ⅱ ) complexes has data within 0.04~0.17 region and the percentage lowering of energy in free gaseous ion (β ° ) has data within 82%~96% which their assigned to the covalency nature of complexes.
| Table 5 Ligand field parameters of the Ni(Ⅱ ) quinolone complexes (Ⅰ — Ⅵ ) |
The experimental effective magnetic moment of the nickel(Ⅱ ) quinoloe complexes (Ⅰ — Ⅵ ) are presence within 3.12~347 BM that agreement with the high spin octahedral geometry (3.00~3.50 BM)[31, 32]. The high spin values may be assigned to the quenched of 3A2g ground state regarding the Ni(Ⅱ ) ion in an octahedral structure[31, 32].
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|