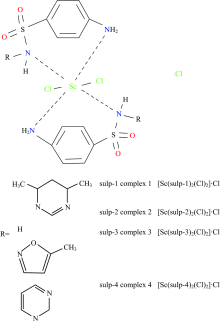
Herein, this article was focused on the synthesis and discussed the spectroscopic characterizations of four new scandium(Ⅲ) sulfa-drug complexes. The nomenclature and symbols of these drugs were sulfadimidine (sulp-1), sulfanilamide (sulp-2), sulfamethoxazole (sulp-3) and sulfadiazine (sulp-4). The microanalytical and spectroscopic analyses which utilized in this study were micro-analyses, magnetic, FT-IR, UV-Vis techniques. The mid infrared spectra deduced that the four sulfa-drug chelates acts as a bidentate chelates with scandium(Ⅲ) ion via two nitrogen atoms of —NH2-Ar and —NH-SO2 groups. Also, the FTIR spectra of Sc3+ complexes referred to the existed of new medium weak bands in the range of 500~400 cm-1 due to stretching vibration bands of ν(M—N). The elemental analysis technique confirmed the 1∶2 stoichiometry between Sc3+ ions and sulp ligand with molecular formula [Sc(sulp)2(Cl)2]·Cl. At room temperature, the results of magnetic measurements for the Sc(Ⅲ) complexes indicated that all of the synthesized complexes have a diamagnetic character with octahedral configuration. The electronic spectra of the free sulfa-drug ligands shows band at 275 and 310 nm which are intraligand charge transfer band. The electronic sbsorption spectra of the Sc3+ complexes were recorded using DMSO solvent. The spectra of complexes display bands within 275~388 nm, which attributed to π—π*, n—π* and charge-transfer M-LCT electronic transitions, which strongly favors the octahedral geometry around Sc(Ⅲ) metal ions.1HNMR spectra of complexes referred to the downfield proton shifts of the —NH2 and NHSO2 groups, which supported the place of coordination. The half maximal inhibitory concentration (IC50) of the ScⅢ complexes was assessed against the human hepato cellular carcinoma (HepG-2) tumor cell line.
Sulfonamides — NHSO2 received much attention because of their tendency to attached with different drugs as a medicinal drugs against bacterial infections and serious diseases in humans[1, 2]. Although sulfonamides are old drugs, they are still considered useful in some therapeutic areas especially in the case of eye infections as well as urinary, gastrointestinal infections[3, 4] and the complex composition of metal ions and sulfa-drugs themselves constitute an important area of research[5]. Moreover, sulfa drugs and their metal complexes have many applications, as well as antibacterial activity such as diuretics, anti-glaucoma or antiepileptic, among other things[6], such as antifungal activity[7], and in many cases, metal compound activity is much better than ligand alone[7]. Fox et al.[8], was discussed number of different metal sulfa drug complexes and their antimicrobial activities. Silver(Ⅰ ), zinc(Ⅱ ) and cerium(Ⅲ ) sulfadiazine complexes were synthesized, characterized and they were found a distinguish properties as a burn treatment[9]. Srivastava et al.[10], have been reported the synthesis and spectroscopic investigations of the coordination between sulphaguanidine ligand and different metal ions like Fe2+, Cu2+, Cd2+, V4+, Mo5+, Pb2+, Se4+, and Sn4+ ions have been synthesized and investigated. The rare earth mixed ligand complexes with sulfamethoxy as a primary and pyridazine as a secondary chelates were synthesized and spectroscopically studied[11], while Gupta and Jha[12] have been characterized an antimony(Ⅲ ) complexes with different kinds of sulfa drugs[13]. The complexation between two sulfa drugs (sulphisoxazole and sulfamethoxazole) ligands and first series of transition metal ions have been synthesized and characterized by Kanagaraj and Rao authors[13].
In literature survey, our group were prepared and discussed some of transition (Mn(Ⅱ ), Hg(Ⅱ ), Cr(Ⅲ ), ZrO(Ⅱ ), VO(Ⅱ )) and rare earth metal (Y(Ⅲ ), Ce(Ⅲ ), Gd(Ⅲ ), Nd(Ⅲ ), Tb(Ⅲ ), Er(Ⅲ )) complexes with sulfasalazine drug ligand[14]. These complexes were spectroscopy characterized and biologically screened against bacteria, fungi and cancer cell lines. In literature survey, there is no work appears to have been done on the chelation between sulfa-drug with ScⅢ ion. It should be noted that the medicinal properties of scandium metal is now recognized, and the scandium has been of interest because of its possible use as an adjunct to 67Ga survey of cancer patients[15]. It was therefore thought to be useful to assemble some Sc3+ sulfa drugs for the first time. The main objective of this work is to prepare and spectroscopic characterize scandium complexes (Ⅲ ) with sulp-1, sulp-2, sulp-3 and sulp-4 sulfa drugs and to determine coordination sites, antimicrobial and anti-cancer assessment.
There are pure grade and used as received, the name of chemicals, exporting company and their purpose can be listed as follows:
A 1.0 mmol (152 mg) of ScCl3 and 2.0 mmol from sulfadimidine (557 mg), sulfanilamide (345 mg), sulfamethoxazole (507 mg), or sulfadiazine (501 mg) were separately dissolved in methanol (25 mL), then mixed and refluxed for 6 hrs, yielding a white solution which was concentrated and left for three days yielding a white precipitate then filtered and washed with hot methanol solvent. The resulting solid precipitates were dried in a dessicator for seven days to afford the desired solid complexes. The elemental, physical and microanalytical data are summarized in Table 1.
| Table 1 Elemental and physical data of sulfa drugs scandium(Ⅲ ) complexes |
1.3.1 Instrument
Perkin Elmer CHN 2400; Jenway 4010 conductivity meter; Bruker FTIR Spectrophotometer; UV2 Unicam UV/Vis Spectrophotometer; Magnetic balance, Sherwood Scientific, Cambridge, England, at Temp 25 ℃; Varian mercury VX-300 NMR Spectrometer.
1.3.2 Measurement
Contents C, H and N; Electrolytic or non-electrolytic character; IR measurements; Electronic spectra; Magnetic moments; 1H-NMR spectrum.
Antimicrobial activity was performed dependent on modified Kirby-Bauer disc diffusion method[16]. The tested species of bacteria and fungi are Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, Aspergillus flavus and Candida albicans. The cytotoxicity of samples was screened against human Hepatocellular carcinoma (HepG-2) using viability assay[17].
2.1.1 Stoichiometric and molar conductance results
The micro analytical data of the synthesized Sc3+ complexes are in agreement with 1∶ 2 stoichiometry (Sc∶ sulp) as refereed in experimental section. The white scandium(Ⅲ ) complexes are stable in static air with highly melting points (> 300 ℃), insoluble in H2O and slightly soluble in DMSO and DMF solvents with gently heating. The ScⅢ complexes have an slightly electrolytic nature[18] with molar conductance values within 34~45 Ω -1· cm2· mol-1 range, this is due to the presence of one chloride ion outside the coordination sphere. The ScⅢ complexes have a diamagnetic state with an octahedral geometry[19] as shown in Fig.1.
2.1.2 Infrared spectral assignments
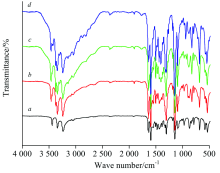
FTIR spectra of ScⅢ sulfa-drug complexes are displayed in Fig.2(a) and the distinguish peaks are assigned in Table 2. Regarding free sulfa-drug ligands, there are observed bands at 3 479~3 423 and 3 379~3 343 cm-1 due to asymmetric and symmetric stretching vibratioo bands — (NH) of aniline group — N
| Table 2 FT-IR spectral frequencies (cm-1) of sulfa-drugs and its ScⅢ complexes |
2.1.3 Electronic and magnetic measurements
An electronic spectra of sulfa-drugs ligands and their Sc(Ⅲ ) complexes were scanned within 200~800 nm range. These spectra have two absorption bands at 275 and 310 nm that attributed to π — π * transition of the aromatic rings[21] and n— π * transition of the anilino — NH2 and sulfonamido — NHSO2 groups. The electronic spectra of the four scandium(Ⅲ ) complexes have different electronic transitions of the π — π * , n— π * and charge-transfer M-LCT are presence at (277, 315, 388 nm), (275, 295, 309 and 372 nm), (276, 281, 297, 298 and 312 nm) and (277, 312, 322 and 326 nm) for the ScⅢ complexes of sulp-1, sulp-2, sulp-3 and sulp-4, respectively. These bands have be relocate rather than free ligands because of binding to the central metal ions. In case of octahedral statement of Sc(Ⅲ ) complex, the charge-transfer transition band is often occur at low energy[21]. The scandium(Ⅲ ) sulfa-drug complexes have μ eff within the range of 0.43~0.69 BM, this is assigned to a diamagnetic octahedral geometry[19].
2.1.4 1H NMR assignments
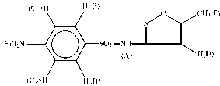
1H-NMR spectra of the sulp-3 drug and its [Sc(sulp-3)2(Cl)2]· Cl complex, were scanned. In Table 3, the signals of sulp-3 drug and its ScⅢ complex with significant shifts are assigned. These results supported the site of coordination between the sulp-3 ligand and ScⅢ metal ions. 1H signals of sulp-3 spectrum (scheme 1) comparing with the spectrum of scandium(Ⅲ ) complex, it is clearly that they are relevant displacement shifted towards downfield when the sulp-3 ligand is coordinated to Sc(Ⅲ ), especially those assigned to 2H; (NH2), that is presented at 6.10 ppm for the free ligand, and at 6.45 ppm in the complex and 1H; (NHSO2) from 10.95 to 11.00, supported that the sulp-3 ligand is chelated via the NH2 anilino nitrogen atom and the — NH sulfonamido nitrogen groups.
| Table 3 1H-NMR proton signals of the sulp-3 ligand and Sc(Ⅲ ) complex |
2.1.5 Antimicrobial and Anticancer assessments
The antimicrobial activities of the free sulfa-drugs com-plexes (sulp-1, sulp-2, sulp-3 and sulp-4) and their Sc(Ⅲ ) complexes have biologically assessed in vitro against bacteria and fungi species (Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, Aspergillus flavus and Candida albicans). The zones of inhibition produced by the test compounds are summarized in Table 4. It is observed that:
| Table 4 Inhibition zone diameter of sulfa-drugs and their Sc(Ⅲ ) complexes against bacteria and fungi species |
(1) [Sc(sulp-1)2(Cl)2]· Cl complex has a significant inhibition against E. coli, B. subtilis, S. aureus and Candida albicans in comparable with free sulp-1 drug.
(2) [Sc(sulp-2)2(Cl)2]· Cl complex has a significant inhibition against P. aeruginosa, B. subtilis, S. aureus, A. flavus and Candida albicans in comparable with free sulp-2 drug.
(3) [Sc(sulp-3)2(Cl)2]· Cl complex has a significant inhibition against E. coli, B. subtilis, S. aureus, Aspergillus flavus and Candida albicans in comparable with free sulp-3 drug.
(4) [Sc(sulp-4)2(Cl)2]· Cl complex has a significant inhibition against E. coli, P. aeruginosa, B. subtilis, S. aureus and Candida albicans in comparable with free sulp-4 drug.
On complexity, the polarity of the metal ion is greatly reduced due to the partial sharing of a positive charge of the metal ion interfering with the donor groups. Increased lipophilicity promote the penetration of complexes in lipid membranes and block mineral binding sites on microorganism enzymes[22].
In vitro cytotoxicity assessment of the [Sc(sulp-4)2(Cl)2]· Cl complex was performed on human hepatocellular carcinoma (HepG-2) tumor cell line. The results evaluated upon the determination of inhibitory concentration of 50% (IC50), the data was listed in Table 5. The [Sc(sulp-4)2(Cl)2]· Cl complex has IC50 equal > 1 000 μ g· mL-1 for HepG-2 cell line. From this data, it is deduced that [Sc(sulp-4)2(Cl)2]· Cl complex has an efficient appropriate against HepG-2 cell line.
| Table 5 Inhibitory efficiency against HepG-2 cell lines for the [Sc(sulp-4)2(Cl)2]· Cl complex |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|