We report on the application of Laser Induced Breakdown Spectroscopy (LIBS) technique to the study of Ca, P, Zn and Sr evolutions for adult caries-affected teeth using nanosecond laser pulses. The aim of this work is to better understand the behaviors of Zn and Sr as trace-elements in the caries eruption with respect to the behaviors of one of the main compounds of the hydroxyapatite crystal which is the calcium. The study was focused on the investigation of these elements’ evolutions from three parts of the enamel surfaces of twenty two adult caries-affected teeth; the healthy part, the dental plaque part and the caries-affected part. The decrease rates of Zn, Sr and P was, also, compared to the Ca one. Comparison concerned normalized emission line intensities. For every species, normalization was done relatively to the emission line intensities of the healthy part. Results showed that abundances of these elements decrease similarly from the healthy parts to the affected-caries parts. The higher decrease rate was noticed for the calcium. The evolutions of Zn and Sr for the three parts of the teeth surfaces cannot inform about the substitution of the calcium by these trace-elements, however the comparison of their decrease rates to the calcium one can be considered as a valuable index of this substitution.
Calcified tissues such as bones, cartilages and teeth are considered as excellent archives of biological matrices due to their interest for elemental analysis in many domains: forensic science[1], geological analysis[2] and preventive dentistry[3, 4]. In dentistry, carious is basically associated with presence of dental plaque in which the amount and the composition are directly related to the health status of the tooth tissues[5].
Several authors[6, 7, 8, 9] were interested in the study of the composition of the dental plaque bacteria. Although these studies give the role of the bacteria on the dissolution of the hydroxyapatite Ca10(PO4)6(OH)2, the role of minerals and trace elements such as P, Na, Sr, Zn, Ba should not be neglected. Indeed, it has been noticed that the matrix elements’ decrease and the non-matrix elements’ increase are usually observed in caries-affected teeth[4]. This is why study of matrix elements such as Ca and P and non-matrix elements such as trace elements can provide information about disease’ s state in case of their lack or excess.
Among used techniques to study these elements in tooth structure, we found Laser Induced Breakdown Spectroscopy (LIBS) technique as a promising technique. It’ s a fast multi-elemental analytical technique where elements are identified by their spectral finger prints[10, 11, 12]. Authors showed that LIBS could be used as a useful tool to quickly identify caries-affected areas in the process of laser drilling or cleaning. They demonstrated that the differences in the LIBS spectra-recorded for healthy, dental plaque and caries-affected parts of the teeth were quite striking.
In this context and by the use of LIBS technique, it has been noticed that Ca is always substituted by one of these minerals and trace elements in hydroxyapatite structure during carious process[3, 13, 14, 15]. In this sense, studies such as[16] have shown that for example, Sr, Ba, and Pb can completely substitute the Ca2+ ions to yield Sr10(PO4)6(OH)2, Ba10(PO4)6(OH)2, and Pb10(PO4)6(OH)2. These substitutions affect the properties of the apatite, for instance incorporation of Sr2+ in apatite increases its solubility. In the same context, it was found[17] that Zn which is readily desorbed from hydroxyapatite by Ca can both reduce enamel demineralization and modify demineralization, during caries clinical trials. Moreover, it has been shown that after caries eruption Zn concentrations apparently increase more on the teeth surface[18]. Also, several other studies have found an inverse relation between caries history and the amounts of Ca and P in dental plaque[19, 20].
In their study[4], researchers used LIBS technique to quantify Ca, Mg, Cu, Zn, Sr, Ti, C, P, H, O, Na and K in healthy and caries part of the tooth specimens. They noticed that the caries part of the teeth contained lower amounts of Ca and P than healthy part and higher concentrations of Mg, Cu, Zn, Sr, C, Na, K, H and O. They have also addressed the role of different metal elements in caries formation. In other works[21, 22] authors presented a spatial mapping of elemental content in tooth samples. Results clearly revealed the changes in the spatial distribution of Sr. Recently[23], LIBS have been used to evaluate the role of F on dentin re-mineralization by studying the possibility of differentiating between non-carious and carious dentin and healthy enamel.
For all those reasons, we have chosen to study Ca, P, Zn and Sr behaviors in carious process, by comparing their emitted line intensities changes between three parts of affected-caries teeth surfaces; the healthy part, the caries-affected region part and the dental plaque region part, by the use of the LIBS technique.
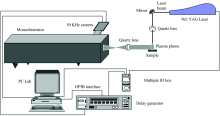
The experimental set-up used in these experiments is shown in figure 1. A standard Nd∶ YAG laser (Brilliant, Quantel), is used to generate a plasma. It runs in its second harmonic at 532 nm, at a repetition rate of 2 Hz. Individual laser pulses had pulse duration of approximately 4 ns. The pulse energy is 30 mJ. One mirror is used to direct the laser beam onto the target surface with an angle of incidence of 45° . The laser light is focused by a fl=10 cm lens to a spot of approximately 1 mm2. The distance between the plasma plume and the entrance slit of the monochromator is 40 cm. The light from the plasma is collected by a focal length fl=10 cm lens placed at the same distance from the plasma plume and the entrance slit of the monochromator, and the image magnification was 1∶ 1. The system used for spectral analysis consisted of a Jobin Yvon THR 1500 monochromator equipped with a 1 200 grooves· mm-1 grating. A fast intensified charge coupled device (ICCD, Andor Technology, model DH520-25F-03) is used for photon detection. Under our experimental conditions, the overall spectral resolution of the system is estimated to be 0.05 nm. Synchronization between the laser and the ICCD detector is ensured by microcomputer via a pulse delay generator (Model DG 535, Stanford Research Systems, Inc). The microcomputer is equipped with software for data acquisition and analysis of spectra. Data acquisition was performed by averaging the signal over ten successive laser shots, and it was verified that the plasma was reproducible by recording the same spectrum several times. The delay time was kept fixed at 1 μ s and the gate width at 1 μ s. The delay time and the gate width were chosen to enhance the signal-to-noise ratio and to decrease the shot-to-shot variations. Studied lines are CaⅡ (210.56 nm), PⅠ (214.23 nm), ZnⅠ (205.26 nm) and Sr(459.99 nm).
Twenty two carious premolars and molars of adults were obtained from a dental clinic. They were cleaned using a toothbrush with distilled water to remove blood on the teeth surfaces. On the enamel surfaces we can easily distinguish three parts, the healthy part, the dental plaque part and the caries part.
For each samples, emission line intensities of the dental plaque part and of the caries part are normalized relatively to the emission line intensities of the healthy part. They are presented in the same scale to be compared (Figure 2). In figure. 2, IH, IDP and IC mean respectively the emission line intensities of the healthy part, the dental plaque and the caries parts.
As it shown in figure 2, we can note a very good similarity between the intensities evolutions of Ca, P, Zn and Sr. Abundances of these species decrease from the healthy part to the caries-affected part. The middle abundances were noticed for the dental plaque part which it’ s the step that precedes the caries affection.
 | Fig.2 Comparison of the normalized emission line intensities for healthy, dental plaque and caries parts (a): Calcium; (b): Phosphorus; (c): Strontium; (d): Zinc |
For the calcium and the phosphorus, which are the main matrix of teeth, this similarity can be explained by the fact that these two elements are the essential compounds of the hydroxyapatite crystal, which is dissolved by the carious eruption. We think this is the reason why they decrease in the same manner. These results are in good agreement with many other works[16, 19, 20, 21].
For the Zn and the Sr, despite they are trace elements, their evolutions are similar to those of Ca and P. they decrease from the healthy part to the caries affected part. We should notice that these results contradict other works [4] and [24], which mentioned that the abundances of Zn and Sr increase just before caries eruption and in the caries-affected part. The analysis of the dental plaque part showed that the amounts of these trace elements are less than those of the healthy part. In fact the dental plaque is the step that precedes the caries eruption. For the caries part their abundances are also less than those the healthy part.
We think that there is probably two ways to interpret these results. The first way is to say that there is no significant positive relationship and correlation between dental caries and these two trace elements[17, 18, 25].
The second way is to consider that our results are not in a disagreement with the well known results previously mentioned[3, 13, 14, 15], which postulate that trace elements such as Pb, Sr, Mg, Mn, Cu, Zn…, substitute the Ca in the hydroxyapatite crystal and increase its solubility during the carious eruption, and we think that when the substitution takes place the crystal will be dissolved and both Ca, P and trace elements will decrease. That’ s why when one analyzes the caries affected parts he will notice that all these species abundances decrease. Nevertheless, one can compare the decrease rates of Sr and Zn relatively to that of Ca. For that we show in figure 3 a comparison of the Ic/IH ratios of Sr and Zn relatively to that of Ca. We notice that Ca has the higher decrease rate relatively to those of Sr aod Zn. One can explain this result by the fact that Ca decreases by two processes; its substitution by trace elements in the hydroxyapatite crystal and its dissolution by the caries eruption.
The aim of this work was the study of Ca, P, Zn and Sr behaviors in caries eruption by laser-induced breakdown spectroscopy (LIBS) technique. The main goal was to try to better understand the evolutions of Zn and Sr in the caries eruption with respect Ca element.
The evolutions of these species were studied for three parts of the surfaces of twenty two caries-affected teeth; the healthy part, the dental plaque part and the caries-affected part. We used normalized intensities of emission lines of these species to compare their abundances in the cited three parts. Results showed that evolutions of all these species are similar; their concentrations decrease from the healthy part to the caries-affected part.
On the other hand, the comparison of the decrease rates of Zn and Sr relatively to Ca, showed that the calcium had the higher one. This result has been explained by the fact that Ca decreases by two processes, its substitution by trace elements in the hydroxyapatite crystal and its dissolution by the caries eruption. For that, Sr and Zn can be considered as precursors of the dental caries eruption.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|