In this work, we have reported the synthesis and spectroscopic characterization of captopril (Cap) coordination compounds: Cu(Cap)·2H2O, Cr(Cap)·H2O, Zn(Cap)·3H2O and Mg(Cap)4. Herein, it is worthily mentioned that the FTIR spectroscopic technique was employed to recognized the nature of coordination between captopril ligand and copper, chromium, zinc and magnesium(Ⅱ) metal ions. In view of the infrared spectroscopic tool, the copper(Ⅱ) metal ion coordinated toward captopril drug ligand through sulfur atom of SH group dependent on the absent of stretching vibration band of —SH. Based on this result, the stretching motion of νa(COO) shifts clearly indicates that Cu2+, Cr2+, Zn2+ and Mg2+ the carboxylic group is employed as coordinative site for all compounds as a metal-ligand coordinative bond. As a general behavior, it is verified that the coordination compound thermal stability (considering the release of captopril molecules, not the release of water molecules) is affected by the metal cation radius: minor radius is associated with higher thermal stability, probably due to a higher metal-captopril bond dissociation enthalpy.
Transition metal complexes remain a very active area of investigation, among other reasons, to the need to understand the chemical behavior of a series of organic molecules towards these metals, taking into account the potential of biological and environmental impacts of these interactions. Hence, the chemical interaction between transition metals and a series of ligands such as caproates[1], mercaptothiazolines and mercaptopyridines[2], cyclic ureas[3], methanesulfonates[4], hexamethylenetetramine[5] and amino acids[6, 7, 8] have been investigated.
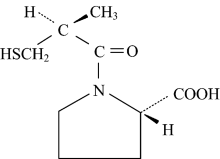
Captopril, 1-[(2S)-3-mercapto-2 methylpropionyl]-Lproline, (also known as “ capoten” ) whose structural formula is shown in Figure 1, is generally employed by the pharmaceutical industry as a blood pressure control agent. However, its interaction with transition metal cations is not so well investigated. Hence, the present work is insert in the above mentioned context and is dedicated to investigated some Cr(Ⅱ ), Cu(Ⅱ ), Zn(Ⅱ ) and Mg(Ⅱ ) captopril coordination compounds.
All reagents were of analytical grade and were employed without further purification. The coordination compounds were synthesized by reaction between the respective metal [Cr(Ⅱ ), Cu(Ⅱ ), Zn(Ⅱ ) and Mg(Ⅱ )] nitrate and captopril aqueous (deionized water) solutions. Cr(Ⅱ )-captopril compound is obtained as a green precipitate. The other compounds were isolated as powders after evaporation at room temperature for five days in a fume hood.
CHN elemental analyses were performed in Perkin-Elmer equipment. The IR vibrational spectra were obtained in KBr discs on a Bruker IF 566 FTIR spectrophotometer. TG curves were obtained in a Shimadzu TG-50 apparatus under nitrogen atmosphere (50 cm3· min-1) at a heating rate of 10 ℃· min-1.
The obtained CHN elemental analysis results and proposed formulas are summarized in Table 1.
| Table 1 Elemental analysis for the synthesized captobril coordination compounds (The calculated values are between parenthesis) |
The main infrared bands for free captopril and coordination compounds are summarized in Table 2. Taking into account the infrared data, can be proposed that to Cu(Ⅱ ) compound there is a coordination involving sulfur, since the characteristic SH stretching band of captopril is absent in the copper compound. Furthermore, the ν a(COO) band of captopril (1 694 cm-1) is shifted to a higher wavenumber (1 724 cm-1) in copper complex.
| Table 2 Main Infrared bands for captopril and its coordination compounds |
On the contrary, to Cr(Ⅱ ), Zn(Ⅱ ) and Mg(Ⅱ ) compounds a coordination involving the COO group can be proposed, based on the downshift observed to the ν a(COO) band. In this context, it is worth noting the fact that the synthesized Zn(Cap)· 3H2O is, probably, different from a structural point of view, of the previously[9] prepared Zn(cap). In a previous study[9, 10, 11] a series of 1:2 metal-Cap compounds (with Co, Ni, Zn, Cd and Cu) were studied and, based on IR, NMR, X-Ray spectroscopy (XPS) and wide angle X-ray scattering (WAXS) techniques, it was conclude that in such captopril compounds the carboxylic group is not involved in the metal-ligand coordination. Despite the fact that in this work the only employed spectroscopic technique was FTIR, the ν a(COO) shifts clearly indicates that for Cu(Cap)· 2H2O, Cr(Cap)· H2O, Zn(Cap)· 3H2O and Mg(Cap)4 the carboxylic group is employed as coordinative site. Moreover, to the 1:1 zinc-captopril compound[9], a chain structure was proposed and, in this case, the carboxylic group was coordinated.
The TG data are summarized in Table 3. The TG curves are shown in Figure 2. Based on the TG data, the following mass loss sequences can be proposed: To all compounds, with exception of Zn(Cap)· 3H2O the first mass loss is associated with the release of physisorbed water molecules. To Mg(Cap)4 is observed the release of captopril molecules in single mass loss. To Cu(Cap)· 2H2O, Cr(Cap)· H2O, and Zn(Cap)· 3H2O, the release of crystallization water molecules is followed by the release of captopril molecules. As a general behavior, it is verified that the coordination compound thermal stability (considering the release of captopril molecules, not the release of water molecules) is affected by the metal cation radius: minor radius is associated with higher thermal stability, probably due to a higher metal-captopril bond dissociation enthalpy.
| Table 3 Thermal analysis (TG) data summery for the synthesized compounds |
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|