The availability of chemical and biological data presented in this paper is the basis for understanding not only the current state of anti-cancer drugs based on gold(Ⅲ), but also the rationale for strategies for future drug design. New Au(Ⅲ) nanosized complexes of cefotaxime (ceph-3) and cefepime (ceph-4) ligands as a 3rd and 4th of cephalosporin generation drugs were synthesized. Gold(Ⅲ) complexes were discussed based on the elemental, molar conductance, thermal and magnetic moment measurements as well as spectral (FTIR,1HNMR, UV-Vis, and XRD) techniques. FT-IR spectra revealed that the ceph-3 and ceph-4 ligands reacted as a bidentate ligands through carboxylate oxygen and β-lactam oxygen groups. The analytical analysis confirm that the molar ratio is 1:1 (Au3+/ceph) with general formula [Au(L)(Cl)2] where L=ceph-3 or ceph-4. The structures of Au(Ⅲ) complexes were presence as a square planar geometry. X-ray diffraction patterns referred to a crystalline nature for all synthesized complexes. TEM analyses confirmed that the synthetic gold(Ⅲ) complexes have a nanosized particles. In vitro antimicrobial activities of Au(Ⅲ) complexes were evaluated towards two types of bacteria (G+ & G-). The antitumor activities of gold(Ⅲ) complexes are appraised against breast (MCF-7) and colorectal adenocarcinoma (Caco-2) cell lines, which means that the two complexes may consider promising anticancer drugs.
Cephalosporins antibiotics are categorized into four generations according to the spectrum of their activity[1]. These are belong to β -lactam antibiotics, the mechanism of action, resistance and other properties are similar to those of penicillin[1, 2, 3]. The first generation of cephalosporins are active toward gram positive bacteria and limited activity against Gram-negative bacteria[4]. Cephalosporins of the second generation show increased activity against Gram-negative microorganisms but are much less active than third and fourth generation agents[2, 3, 4, 5, 6]. Mn(Ⅱ ), Co(Ⅱ ), Ni(Ⅱ ), Cu(Ⅱ ) and Cd(Ⅱ ) complexes of cefotaxime were synthesized with molar ratio M:L (1:2). The place of coordination take place through the oxygen of carboxylate, oxygen of beta-lactam ring and nitrogen of aminothiazole ring[7]. Cefepime has been described as the fourth generation of broad-spectrum antibiotics[8, 9]. It is effective against some bacteria that fight other antibiotics and is used to treat Gram-negative and Gram-positive bacteria, especially those that cause infections in the lungs, kidneys, bladder, skin, and abdomen[10, 11]. Cefepime was reacted with some of transition metal(Ⅱ ) ions and gave a 1:1 (M:L) complexes, which were discussed using a physicochemical and spectroscopic analyses[12]. Many metal-based compounds have been manufactured with promising anti-cancer properties, some of which are already using in clinical practice for diagnosis and treatment while some are undergoing clinical trials[13, 14, 15, 16].
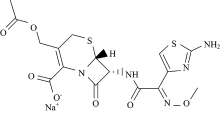
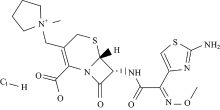
In view of this point and as part of metal-drug interactions[17, 18, 19, 20, 21], we reported here the synthesis, spectroscopic characterizations and biological investigations of gold(Ⅲ ) complexes of a cefotaxime (ceph-3) and cefepime (ceph-4) drugs (Fig.1).
The chemicals AuCl3, cefotaxime sodium and cefepime hydrochloride were received from Aldrich chemical company (United States) and used without further purification. The solvents were used have an analytical grade.
A dissolved solutions of cefotaxime sodium and cefepime hydrochloride (1 mmol in 50 mL CH3OH) were mixed with AuCl3 (1 mmol in 20 mL CH3OH). The brown solutions were neutralized at pH 7~8 by 0.1 mol· L-1 of ammonia solution, then refluxed for 3 hrs with continuous stirring. The solid brown precipitates were isolated, washed with few amount of methanol solvent and dried under vacuum. Single crystals for X-ray structural studies were unsuccessful.
| Instrument | Measurement |
|---|---|
| Perkin Elmer CHN 2400 | Contents C, H and N |
| Jenway 4010 conductivity meter | Electrolytic or non-electrolytic character |
| Bruker FTIR Spectrophotometer | IR measurements |
| UV2 Unicam UV/Vis Spectrophotometer | Electronic spectra |
| Magnetic balance, Sherwood Scientific, Cambridge, England, at Temp 25 ℃ | Magnetic moments |
| Varian mercury VX-300 NMR Spectrometer | 1H-NMR spectrum |
| Shimadzu TGA-50H | Thermal analysis |
| X ’ Pert PRO PAN analytical XRD | X-ray diffraction patterns |
| Quanta FEG 250 equipment | Scanning electron microscopy (SEM) images |
| JEOL 100s microscopy | Transmission electron microscopy images (TEM) |
Antimicrobial and anticancer tests: The antimicrobial test of the Au(Ⅲ ) cephs complexes was scanned against (G(+)(Staphylococcus Epidermidis and Staphylococcus Aureus) and G(-)(Klebsiella and Escherichia coli) bacteria dependent on modified Kirby-Bauer method[22, 23]. The anticancer assessments of gold complexes against Colorectal adenocarcinoma (Caco-2) and Breast cancer (MCF-7) cell lines were performed upon the standard neutral red uptake assay[24].
Two synthesized gold(Ⅲ ) complexes are insoluble in more organic solvents but dissolved in two mentioned solvents e.g. (DMF and DMSO) and melting with decomposition at the temperature range -250 ℃. The stoichiometry and analytical data of the complexes reveal that the molecular formula of the compositions as [Au(ceph-3)(Cl)2] (1) and [Au(ceph-4)(Cl)2] (2). At room temperature the conductivity data was measured in DMSO and the results showed that the prepared complexes have a non electrolytic property (25~29 ohm-1· cm2· mol-1)[17] due to the presence of chloride ion inside the coordination sphere. From the TGA curves, the synthesized complexes don’ t recorded any mass loss up to 120 ℃, this assigned to the absent of a crystalline water molecules. The microanalytical and stoichiometric results of the complexes are listed in Table 1.
| Table 1 Elemental and physical data of gold(Ⅲ ) ceph-3 and ceph-4 complexes |
Both cefotaxime and cefepime ligands contains the — NH, — COOH, — NHCO and C=O functional groups and its molecular model reveals that its structure is able for complexation. The characteristic infrared spectral bands of ceph-3, ceph-4 free ligands and their gold(Ⅲ ) complexes are assingend in Table 2. The stretching vibration band of ν (C=O) β -lactam ring is exist at 1 757 and 1 771 cm-1 in the spectra of ceph-3 and ceph-4, respectively[25], this band is absence in the IR spectra of Au(Ⅲ ) complexes. In case of the free ceph ligand, the ν (CO) of amido group is presence at ~1 640 cm-1, while after chetation with gold(Ⅲ ) ion, this band is detectable as the same frequency with not significant shift. Therefore the coordinationbetween gold(Ⅲ ) ion and ceph-3 or ceph-4occurs through the oxygen atom of β -lactam rather than oxygen of amido group. The asymmetric stretching frequency of carboxylate group ν asCOO which is presence in free ceph-3 and ceph-4 at 1 531 and 1 560 c
| Table 2 FT-IR frequencies and assignments of the ceph-3, ceph-4 free ligands and their gold(Ⅲ ) complexes |
1HNMR spectrum of ceph-3 drug ligand is assigned (Table 3) as: δ (ppm) 1.99 (3H; — COOCH3), 3.28~3.58 (2H; S— CH2), 3.91 (3H; N— O— CH3), 4.63~4.79 (2H; CH2— O— CO), 5.11 (H; N— CHβ -lactam), 5.71 (H; CO— CHβ -lactam), 6.89 (H; Ph5-member ring), and 9.03 (H; CONHamido group)[27]. Regarding 1HNMR spectrum of [Au(ceph-3)(Cl)2] complex, the characteristic bands of β -lactam and the chemical shifts of six-remembered ring attached with carboxylate group!are moved to downfield δ (ppm). The 1HNMR spectrum of the Au(Ⅲ ) complex changed in compared with the represented ceph-3 ligand and the signals are present with downfield, as expected, due to increased conjugation on coordination[28].
| Table 3 1H-NMR spectral data of ceph-3 drug and its gold(Ⅲ ) complex |
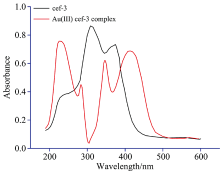
The electronic spectra of ceph-3 and ceph-4 in DMSO solvent within the UV-Vis region were scanned. These spectra of free ligands have three absorption bands at wavelengths 237, 315 and 386 nm due to π -π * , n-π and intra-ligand charge transfer bands[25, 28]. The absorption spectra of gold(Ⅲ ) complexes (Fig.2) corresponding to an electronic transition band at 240 nm, which is assigned to the absorption band of 1A1g (D)→ 1Eu(D), while the band at 430 nm is due to the electronic transition of 1A1g(D)→ 1A2g (D). These absorption bands are revealed that the gold(Ⅲ ) complexes of ceph-3 and ceph-4 have a square planar geometry[29]. The diamagnetic character of the synthesized gold(Ⅲ ) complexes revealed that the presence of complexes in a square-planar configuration.
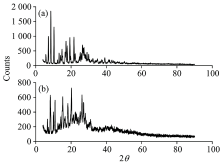
The XRD values of gold(Ⅲ ) complexes as referred in Figure 3(a, b) within range of 5° ~50° (2θ ). According to Figure 3, the 2θ values with maximum intensity of the peaks for Au(Ⅲ ) complex are observed to be 2θ =10.30° , 13.18° , 24.73° , 25.84° , 26.98° , 36.10° , and 37.39° which corresponds to d=8.581 4, 6.712 0, 3.597 1, 3.445 1, 3.302 1, 2.486 1, and 2.403 2, respectively. All these peaks calculated on the obtained values of interplanar distance are compared with the recorded ones[30]. This implies that gold(Ⅲ ) complex has square-planar geometry in structure. The X-ray diffraction patterns of gold(Ⅲ ) complexes deduced the crystalline structure with nano size (20~25 nm) based on Debye-Scherrer equation[31].
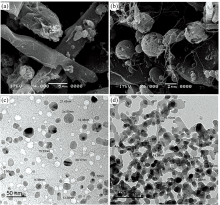
The SEM micrograph [Fig.4(a, b)] reveals the overall appearance of Au(Ⅲ ) ceph-3 and ceph-4 complexes. The particles are nearly rod or spherical in shape have uniform size and distribution with asymmetrical sizes, might be due to distribution of temperature during synthesized. It can be observed that product aggregation is constituted by many irregular particles with a variety of particle size due to the difference chemical structure of ligands. TEM images of Au(Ⅲ ) complexes are shown in Figure 4(c, d), with a nanoscale range between 20~24 nm in agreement with XRD data.
The proposed structures of the synthesis gold(Ⅲ ) complexes concluded that the coordination of ceph-3 and ceph-4 occurs through the carboxylate and lactam carbonyl oxygen atoms as mentioned in Figure 5.
The susceptibility of strains of bacteria “ G(+)(Staphylococcus Epidermidis and Staphylococcus Aureus) and G(-)(Klebsiella and Escherichia coli)” towards cefotaxime (ceph-3) and cefepime (ceph-4) ligands and their gold(Ⅲ ) complexes were screened by measuring the size of inhibition diameter (Table 4). The cefotaxime and cefepime antibiotic drugs and the gold(Ⅲ ) complexes have a bactericide diameters > 19 mm with highly sensitive[32, 33]. Generally, gold(Ⅲ ) complexes [Au(ceph-3)(Cl)2] and [Au(ceph-4)(Cl)2] were found to have higher antibacteria activities than that of corresponding ligands. The highest antibacterial activity was showed by the [Au(ceph-3)(Cl)2] complex against Escherichia coli being inhibition zone diameter about 29 mm higher than ceph-3 activity.
| Table 4 Inhibition zone (mm) of ceph-3 drug, ceph-4 drug, Au3+ ceph-3 complex and Au3+ ceph-4 complex |
The anticancer activities of gold(Ⅲ ) complexes of ceph-3 and ceph-4 drugs against the growth colorectal adenocarcinoma (Caco-2) and breast cancer (MCF-7) cell lines are listed in Table 5. The % cell inhibition and IC50 values (26.1, 110, 1.99, and 11.4 μ g· mL-1) for the Au3+ ceph-3 complex against (Caco-2), Au3+ ceph-3 complex against (MCF-7), Au3+ ceph-4 complex against (Caco-2) and Au3+ ceph-4 complex against (MCF-7), respectively. They indicates that gold(Ⅲ ) complexes have a sensitivity towards the Caco-2 and MCF-7 cancer lines in comparable with cisplatin standard drug (5.71 and 3.67 μ g· mL-1).
| Table 5 Sample concentration and cell viability of Au3+ complexes and cisplatin against (Caco-2) and (MCF-7) cancer cell lines |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|