作者简介: 张辰凌, 1986年生, 中国地质科学院水文地质环境地质研究所工程师 e-mail: zhangchenling2011@126.com
重金属污染是一个相当严重的环境问题。 镉具有很强的生物毒性和不可降解性, 对生态环境和人体健康有极大威胁, 被列为优先控制污染物。 环境中镉的主要污染源是电镀、 采矿和化学工业等部门的废水, 如何简单高效去除水中的镉, 有重要的社会意义和经济意义。 目前, 水中重金属的去除方法有化学沉淀、 膜分离、 离子交换、 吸附、 电解等, 其中吸附法因简单高效而广泛应用。 活性炭纤维是一种新型活性炭, 孔径小且均匀, 表面官能团发达, 吸附性能好, 逐步应用于水处理领域。 以电感耦合等离子体光谱为检测手段, 佐以比表面积分析, X射线衍射, 元素分析和傅里叶变换红外光谱, 研究比较了三种活性炭纤维(纤维炭网、 活性炭无纺布、 活性炭纤维毡)的结构特点及其对水中镉的吸附性能。 三种活性炭纤维结构基本类似, 具有较发达的孔隙结构。 活性炭无纺布极性较强, 表面有丰富的羟基、 羧基、 醛基等含氧官能团, 对水中镉的吸附作用最大。 因此, 选择活性炭无纺布为吸附剂进行后续实验。 研究了活性炭无纺布吸附镉的影响因素, 如溶液pH, 吸附时间等。 溶液pH影响吸附剂表面电荷及水中镉的存在状态。 水中镉的去除效率随溶液初始pH的增大而增大, 在较低pH时, 吸附剂与Cd2+间存在静电斥力, 同时H+和Cd2+存在竞争吸附, pH>9时, 镉的去除是吸附与沉淀协同作用的结果, 选择pH为6~7。 在吸附的初始阶段, 活性炭无纺布对Cd2+的吸附量迅速增加, 10 min时, 吸附率达到72%。 随着吸附位点逐渐被Cd2+所填充, 吸附速率逐渐变慢, 300 min时, 吸附容量基本无变化, 吸附趋于平衡。 优化了镉的吸附条件后, 进行等温吸附实验和动力学实验。 结果表明, 25 ℃时, 吸附时间为300 min, pH 6.0条件下, 当镉的平衡浓度在20.00 mg·L-1时, 活性炭无纺布对镉的单位质量吸附量和单位比表面积吸附量分别是3.04 mg·g-1和0.035 mg·m-2。 用Langmuir方程( R2=0.997, KL =1.796 L·mg-1)和Freundich方程( R2=0.895, KF=0.918 L·mg-1, n=2.12)拟合活性炭无纺布对镉的等温吸附数据, Langmuir方程计算的理论吸附量为3.07 mg·g-1, 与实验值相当, 并且线性系数更高, 说明该体系的吸附符合Langmuir方程, 主要为单分子层吸附。 Langmuir分离因子介于0和1之间, 表明活性炭无纺布对镉的吸附容易进行。 用准一级动力学方程、 准二级动力学方程、 颗粒内扩散方程和Elovich方程四种动力学模型拟合吸附过程。 在吸附的前5 min, 镉在活性炭无纺布上的吸附符合颗粒内扩散方程( R2=0.985), 吸附主要受颗粒内扩散控制。 在吸附的5~300 min, 颗粒内扩散方程拟合较差。 整个吸附过程符合准二级动力学方程( R2=0.999, k2=0.367 g·mg-1·min-1), Elovich方程( R2=0.981, a=0.271 mg·g-1, b=0.083 mg·g-1·(lg min)-1)和准一级动力学方程( R2=0.927, k1=0.008 8 min-1)次之, 颗粒内扩散方程( R2=0.785)最差。 活性炭无纺布对镉的吸附过程是一种化学作用为主的吸附过程。 对5.00 mg·L-1含镉水样, 活性炭无纺布投放量为10 g·L-1时, 吸附后水中镉的浓度小于0.10 mg·L-1, 符合《污水综合排放标准》(GB 8978—1996)。 活性炭无纺布可同时吸附镉, 铜, 铅, 铬等重金属离子, 选择性较差。 但在电镀、 采矿等实际废水中重金属种类复杂, 适当提高吸附剂投放量, 可同时去除多种重金属。 利用活性炭无纺布吸附处理含镉水样, 处理效果好、 操作简单, 可以作为去除水中镉的吸附剂, 为含镉废水的处理提供了技术支持和理论基础。
Heavy metal contamination has become one of the most serious environmental problems. Among these elements, cadmium (Cd) is regarded as one of the major priority pollutants in drinking water due to its high biotoxicity, non-biodegradability and persistence in the whole environment. It has hazardous effects on ecological environment and human health. The major sources of cadmium release into the environment are waste water from electroplating factories, mining activities, chemical manufacturing processes and refining processes. It is of great social significance and economic benefits to study how to remove cadmium in water/wastewater friendly and efficiently. The commonly traditional methods used for the removal of cadmium from waste water include chemical precipitation, cementation, membrane separation, ion exchange, solvent extraction, adsorption process and the like processes. Among these methods, adsorption process is generally preferred and widely used because of its high efficiency, low cost, simplicity and availability. Activated carbons are well-known adsorbents extensively used for effluent treatment in many industrial processes. Activated carbon fiber (ACF) that has the advantages of uniform micropore structure, well-developed functional groups and good adsorption properties, is a new type of activated carbon, and has been gradually applied in water/wastewater purification systems. In this study, a comparative adsorption analysis of three activated carbon fibers (net, none-woven fabric and felt) was carried out and characterized with inductively coupled plasma optical emission spectrometry (ICP-OES) as the analysis method. Several characterization techniques (specific surface area analysis, X-ray diffraction, Fourier transform infrared spectroscopy and elemental analysis) were also employed to identify the structures of three activated carbon fibers. The structures of three fibers were similar with well-developed micropore structure according to X-ray diffraction and specific surface area analysis. The activated carbon none-woven fabric fiber has the strongest polarity and the highest oxygen content in the three ACFs. And it has a lot of oxygen-containing functional groups on the surface such as hydroxyls, carboxyls and aldehydes. Besides, the adsorption of cadmium on activated carbon none-woven fabric fiber obtained the most satisfied result with 97% removal efficiency. So that none-woven fabric was chosen as the target adsorbent in the subsequent experiments. The adsorption property of cadmium ions on none-woven fabric and its influential factors such as initial solution pH value and contact time between cadmium ions and the adsorbent were examined and optimized in the next procedure. The removal efficiency and adsorption capacity vary with pH value because pH affects not only the surface charge characteristics of activated carbon fiber but also the chemical forms of cadmium in the aqueous solution. The adsorbent charge turned into negative with an increase of pH value, which is favorable for removal of cadmium due to the emerging electrostatic attractions between cadmium ions and the oxygen-containing functional groups. The removal efficiency of cadmium increased with the increase of the initial pH of the solution. Cadmium removal efficiency by none-woven fabric increased significantly in the pH range of 1~6, and slightly from pH 6 to 9. Cadmium removal efficiency from 98.04% to 99.81% was obtained from pH 6~9. At lower pH, there was electrostatic repulsion between adsorbent and cadmium ions and competitive adsorption between H+ and Cd2+. The maximum uptake value was achieved at pH>6, which might be attributed to the presence of lone pair of electrons on oxygen atoms that are beneficial to coordinate with cadmium ions to give the corresponding complex compounds. At pH>9, the removal of cadmium was the synergistic effect of adsorption on adsorbent, complex formation and precipitation formation. Based on pH influence study, the pH value of solution was adjusted to 6~7 in subsequent adsorption experiments. In the initial adsorption stage, cadmium adsorption ratio increased rapidly with the increased contact time, and 72% of cadmium was removed in the first 10 min. Then the adsorption rate lowered down with the adsorption sites of none-woven fabric filled with cadmium ions. And finally, adsorption efficiency was up to a constant after 300 min, and the adsorption capacity reached a dynamic equilibrium. Further, any increase of contact time did not show any considerable changes in percent removal of cadmium. After the adsorption conditions were optimized, the isothermal adsorption experiment and kinetic experiment of cadmium were carried out subsequently. The results showed that the saturated adsorption capacity of cadmium on none-woven fabric fiber were 3.04 mg·g-1 and 0.035 mg·m-2 when the equilibrium concentration of Cd2+ was 20.0 mg·L-1, pH was 6.0, and the adsorption time was 300 min at 25 ℃. As the concentration of cadmium in the solution continued to increase, the adsorption amount tended to be a dynamic balance. The isothermal adsorption data was simulated by Langmuir model and Freundlich model. The main assumption of Langmuir isotherm is the monolayer formation of the solute on the surface of adsorbent without interaction between the solute molecules. The Freundlich isotherm is most commonly used to explain adsorption on a surface having heterogeneous energy distribution. In Langmuir model, the linear correlation coefficient was 0.997, and Langmuir factor was 1.796 L·mg-1. In Freundlich model, the linear correlation coefficient was 0.895, and Freundlich factor was 0.918 L·mg-1, and n was 2.12. The linear correlation coefficient of Langmuir model was much higher than that of Freundlich model. The adsorption capacity calculated according to the Langmuir model was 3.07 mg·g-1, which was just approximated to the experimental data of 3.04 mg·g-1. It indicated that the adsorption system conformed to the Langmuir equation better, and it dominated that adsorption on none-woven fabric was based on monolayer adsorption. The value of Langmuir separating factor was estimated for the entire concentrations range and it was calculated between 0 and 1, confirming favorable cadmium adsorption condition. In order to investigate the adsorption processes of cadmium on none-woven fabric, the kinetic data were fitted by four dynamic models, which were pseudo-first-order kinetic equation, pseudo-second-order kinetic equation, intra-particle diffusion equation and Elovich equation. In the first 5 min of the kinetic process, the adsorption capacity of cadmium accorded with intra-particle diffusion equation fairly since the linear correlation coefficient was calculated 0.985. It indicated that the diffusing rate in the interior surface layer of the particle was the control?step in the first 5 min. However, in the 5~300 min, the adsorption kinetic data could not accord with that equation at all. The whole adsorption process of cadmium on activated carbon none-woven fabric fiber accorded with the pseudo-second-order kinetic equation approximately with linear correlation coefficient 0.999 and reaction rate constant 0.367 g·mg-1·min-1. And the adsorption capacity obtained by calculation of pseudo-second-order kinetic equation was just approximated to that obtained by experiment. The whole adsorption process accorded with the Elovich equation and the pseudo-first-order kinetic equation relatively lower. In the Elovich equation, the linear correlation coefficient was 0.981, and Elovich factors were 0.271 mg·g-1 and 0.083 mg·g-1(lg min)-1. In the pseudo-first-order kinetic equation, the linear correlation coefficient was 0.927, and reaction rate constant was calculated to be 0.008 8 min-1. And the adsorption capacity obtained by calculation of pseudo-first-order kinetic equation differed from the experimental data. So that adsorption of cadmium on ACF was based on chemical reactions, such as electrostatic interaction and hydrogen bond. In removal of heavy metals by none-woven fabric, taking a 5.0 mg·L-1 cadmium contained synthetic wastewater sample for example, the cadmium content was less than 0.10 mg·L-1 after adsorption by none-woven fabric fiber, and it met Integrated Waste Water Discharge Standard (GB 8978—1996). Besides cadmium, heavy metals such as copper, lead and chromium could be adsorbed by none-woven fabric with removal efficiency higher than 95%. None-woven fabric had poor selection of cadmium adsorption in water with variety of heavy metals. When it met electroplating waste water and mining waste water, various heavy metals could be removed with more adsorbent added in adsorption treatment. The results showed that it was suitable, simple and effective in treating water containing cadmium by activated carbon fiber due to its good effect and convenient operation. And this study provided technical assistance and theoretical support in real waste water treatment.
镉具有毒性强、 易富集、 难降解等特征, 对生态环境和人体健康都有极大威胁[1, 2]。 环境中镉主要污染源是电镀、 采矿和化学工业等部门排放的废水[3]。 因此, 其排放前的防治成为重点。 我国《污水综合排放标准》(GB 8978— 1996)中规定了镉为一类污染物, 其排放浓度不得超过0.10 mg· L-1。 研究如何去除水中的镉, 有重要的社会意义。 目前, 常用的重金属离子去除方法有化学沉淀、 吸附、 电解、 离子交换和膜分离等[4, 5, 6, 7, 8], 其中, 吸附技术[9, 10, 11, 12]由于具有快速、 高效而广泛应用于重金属的去除。 活性炭纤维(activated Carbon Fiber, ACF)[13, 14, 15]是一种新型活性炭, 具有比表面积大, 孔径均匀, 表面官能团发达等优点, 而且价廉易得, 逐步应用于水处理领域。
本研究对比了活性炭无纺布、 纤维炭网、 活性炭纤维毡对水中镉的吸附, 选用活性炭无纺布为吸附剂。 分析了其吸附等温模型和吸附过程动力学模型, 提供了关键性能参数, 明确了活性炭无纺布吸附水中镉的可行性。
镉标准贮备液(中国计量科学研究院); 硝酸、 氢氧化钠(优级纯, 天津市科密欧化学试剂有限公司); 纤维炭网、 活性炭无纺布、 活性炭纤维毡(南通森友炭纤维有限公司)。
电感耦合等离子体光谱仪(Optima 8000, 美国PerkinElmer公司); 红外光谱仪(Frontier FTIR, 美国PerkinElmer公司); 元素分析仪(FLASH EA1112, 美国Thermo Fisher Scientific公司); 全自动比表面积及孔隙度分析仪(3H-2000PS2, 北京贝士德公司); X射线衍射仪(XRD-6100X, 日本岛津公司)电子天平(PWC254, 英国ADAM衡器公司); 恒温振荡器(ZD-85, 苏州国华仪器有限公司)。
射频功率: 1 400 W; 冷却气流量: 12 L· min-1; 辅助气流量: 0.2 L· min-1; 雾化气流量: 0.85 L· min-1; 观测方向: 轴向; 观测高度: 15 mm; 波长: 228.8 nm。
1.3.1 起始溶液pH影响实验
将5 mg· L-1的含镉溶液用0.01 mol· L-1的HNO3和NaOH溶液调节至pH 1~9, 活性炭无纺布投加量为300 mg, 于25 ℃下恒温振荡, 每组实验做两组平行。 吸附平衡后取样, 过滤、 酸化后用ICP-OES测定浓度。
1.3.2 等温吸附实验
将300 mg活性炭无纺布与不同浓度的含镉溶液混合, 于25 ℃下恒温振荡, 含镉溶液的浓度分别为0.1, 0.2, 0.5, 1.0, 1.5, 2.0, 5.0, 10.0, 20.0, 30.0, 40.0, 50.0和80.0 mg· L-1, 每组实验做两组平行。
1.3.3 动力学实验
配制一系列5 mg· L-1 的含镉溶液, 将30 mL的溶液分别装入具塞玻璃锥形瓶中, 在溶液里分别加入300 mg活性炭无纺布, 于25 ℃下进行恒温振荡, 每隔一定时间取样, 每组实验做两组平行。
三种活性炭纤维的X射线衍射谱均有三个峰, 强衍射峰在2θ =17° 附近, 弱衍射峰在2θ =14.1° 和2θ =25.7° 附近, 可知三种活性炭纤维结构基本相似, 都属无定形碳。 三种活性炭纤维均具有较发达的孔隙结构, 活性炭无纺布和纤维炭网比表面积相近, 小于活性炭纤维毡。 活性炭无纺布的比表面积为85.93 g· m-2。 三种活性炭纤维的元素组成见表1, 活性炭无纺布碳含量最低, 氧含量最高, 极性最大。 三种活性炭纤维的红外谱图见图1。 活性炭无纺布在896~1 358 cm-1处有大强度的吸收峰, 分子具有强极性基团; 纤维炭网和活性炭无纺布在3 100~2 900 cm-1处有明显的吸收峰, 归属为自由或缔合— OH的伸缩振动; 三种活性炭纤维都在1 734~1 742 cm-1处出现C=O的伸缩振动峰。 综上所述, 活性炭无纺布可能有较多羧基、 羟基、 醛基等官能团。
| 表1 活性炭纤维的元素组成和原子比 Table 1 Elemental compositions and atomic ratios of the ACFs |
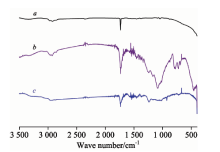 | 图1 三种活性炭纤维的红外图谱 a: 纤维炭网; b: 活性炭无纺布; c: 活性炭纤维毡Fig.1 FTIR spectra of three ACFs a: Activated carbon fiber net; b: Activated carbon fiber none-woven; c: Activated carbon fiber felt |
在含镉溶液里分别加入纤维炭网、 活性炭无纺布、 活性炭纤维毡, 其吸附效率分别为16.38%, 97.46%和59.23%, 因此选用活性炭无纺布进行后续试验。
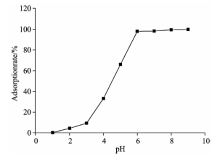
从图2可以看出吸附效率随pH的增大而增大。 在pH< 5时, 镉去除率较低, 吸附剂与Cd2+间存在静电斥力, pH越小, 斥力越大。 当pH 6~7时, 静电斥力减小, 吸附量逐渐增大。 当pH继续增大, CdOH+和Cd(OH)2形态逐渐增加, 镉的去除是吸附与沉淀协同作用的结果。 为了考察pH对吸附的影响, 取pH小于临界pH 9.04。 因此后续试验溶液均调节pH 6左右。
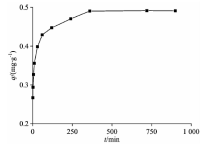
由图3可见, 在吸附的初始阶段, 活性炭无纺布对Cd2+的吸附量迅速增加, 10 min时, 吸附量达到72%。 随着吸附位点逐渐被Cd2+所填充, 吸附速率逐渐变慢, 吸附趋于平衡, 在300 min后, 吸附容量基本无变化, 吸附趋于平衡, 吸附后水中镉的浓度小于0.10 mg· L-1, 符合《污水综合排放标准》(GB 8978— 1996)。
活性炭无纺布对镉的吸附量随镉平衡质量浓度的增加而增加, 当镉的平衡浓度在20.0 mg· L-1时, 吸附量为3.04 mg· g-1。 活性炭无纺布对镉的吸附等温线经Langmuir方程和Freundich方程模型拟合参数见表2。 可以看出, Langmuir方程的R2比Freundich方程高, 表明该体系的吸附主要为单分子层吸附。 在25 ℃下, 活性炭无纺布对镉的饱和吸附量理论值为3.07 mg· g-1, 与实验值吻合。 分离因子RL是一个与Langmuir等温线基本特性有关的常数, 在25 ℃下, RL值都介于0到1之间, 表明活性炭无纺布对镉的吸附容易进行。
| 表2 镉的等温吸附参数 Table 2 Parameters of Langmuir and Freundich isotherms for Cd |
吸附剂比表面积是镉离子吸附的重要影响因素, 活性炭无纺布的比表面积不大, 以单位面积为基准计算, 其吸附量为0.035 mg· m-2, 与污泥活性炭[10]和微米碳纤维[11]相当, 小于纳米二氧化硅材料[12]。
为深入探讨活性炭无纺布对Cd2+吸附的动力学特征, 分别运用准一级动力学方程、 准二级动力学方程、 颗粒内扩散方程和Elovich方程四种动力学模型来计算, 结果见表3。 在25 ℃下当吸附时间在0~5 min时, qt与t1/2线性相关(R2=0.981), 主要受颗粒内扩散控制。 对整个吸附过程, 准二级动力学方程拟合度最好, 其R2为0.999, Elovich方程和准一级动力学方程次之, 颗粒内扩散方程最差。 用准二级动力学模型求解的吸附量与实验值无显著偏差, 是一种化学作用为主的吸附过程。
| 表3 活性炭无纺布对镉的吸附动力学方程拟合特征值 Table 3 Adsorption kinetics parameters of Cd on the ACF |
(1)实验对比了活性炭无纺布、 纤维炭网、 活性炭纤维毡三种活性炭纤维对水中镉的吸附效果, 选用活性炭无纺布为吸附剂。
(2)25 ℃下, 吸附热力学结果显示吸附过程符合Langmuir模型, 说明该体系的吸附主要为单分子层吸附。
(3)吸附动力学符合准二级动力学方程, 是一种化学作用为主的吸附过程。
(4)对5.0 mg· L-1含镉水样, 活性炭无纺布投放量为10 g· L-1时, 吸附后水中镉的浓度小于0.10 mg· L-1, 符合《污水综合排放标准》(GB 8978— 1996)。
(5)活性炭无纺布可同时吸附水中Cd2+, Cu2+, Pb2+, Cr6+, 选择性较差, 但该吸附剂廉价易得, 稳定性好, 且实际废水中重金属种类复杂, 适当提高投放量, 可同时去除多种重金属。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|