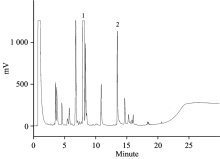
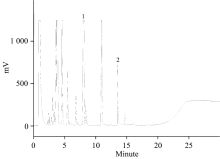
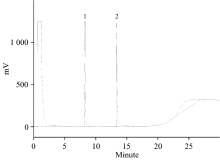
牛樟精油具有独特浓郁的香气, 其中以α-松油醇(
Stout camphor essential oil has a special scent due to its content of specific aromatic terpenoid compounds. Essential oils prepared from different species, varieties or subspecies have been shown to possess varied compounds. Of the major essential oil components extracted from the Stout Camphor tree, α-terpineol has been shown to be a key component, while essential oils prepared from other camphor species may not contain this terpene alcohol. Therefore, α-terpineol can be used as an index element to represent the purity of Stout Camphor essential oil. However, a method that can quantitatively examine the quality and purity of commercial Stout Camphor essential oils is necessary. By adding an internal standard to essential oil samples, this study aimed to develop a simple and reliable method for determining the level of α-terpineol in Stout Camphor essential oils on the market. Capillary column gas chromatography has the advantages of high resolution and high sensitivity, and is still one of the most important spectral analysis techniques in modern times. Therefore, this study developed a rapid method that used vanillin as an internal standard to determine the level of α-terpineol, a key component of the essential oil extracted from the stout camphor tree, using gas chromatography. The analysis of each sample only required 30 min. The lowest limit of quantitation was as low as 1 μg·mL-1. Add α-terpineol 1.0 and 10.0 mg to commercially available Cinnamomum micranthum Hayata essential oil and stout camphor wood essential oil, and the recovery rates were more than 98% (98%~103%) with a coefficient of variation below 10.8%. We then analyzed 15 commercially-available essential oil samples and one essential oil sample directly extracted from stout camphor wood. We found that the levels of α-terpineol in these samples were within the range of 21.3%~51.6%. In conclusion, this method has a high accuracy, and the α-terpineol levels can be used as an index to rapidly determine the quality of the stout camphor essential oil on the market.
Camphor laurel (Cinnamomum camphora (
Stout camphor (C. kanehirai Hayata), one of the five major broadleaf trees in Taiwan, is also a unique native tree of Taiwan[4]. Stout camphor wood has a fine and uniform texture, and is ideal for high-grade furniture and suitable for carving. Its market price is high, approximately US$ 3 500 to US$ 10 000 per ton. An endemic fungi species that only grows on the stout camphor tree in the wild, called stout camphor fungus (Antrodia cinnamomea), has been used as a traditional natural medicine with anti-cancer and liver protection properties in Taiwan for many decades. Recent laboratory and clinical studies have demonstrated that extracts of A. cinnamomea possess bioactivities including anti-cancer and vasorelaxation, and many more other medicinal applications have been identified in the past few years[5, 6, 7, 8, 9, 10]. These developments have increased the demand for A. cinnamomea, and methods are being developed that will enable large-scale growth of fruiting bodies of A. cinnamomea, including stout camphor wood, indoors. This has increased the demand for stout camphor wood, and has led to the number of stout camphor trees being significantly reduced. Therefore, the stout camphor tree has been listed as an endangered plant by the Taiwan government, and felling and trading of wild stout camphor trees are not permitted.
Therefore, as stout camphor wood is in short supply, it has become very valuable, and illegal logging is increasing. Recently, different methods of culturing A. cinnamomea using wood from other camphor species, or using media to grow fruiting bodies of A. cinnamomea in solid-state or liquid-state cultures, or mycelia of the fungi in petri dish cultures, have been reported[11, 12, 13, 14]. Some studies have shown that adding essential oil extracted from the stout camphor tree to the medium culture significantly improved the growth of A. cinnamomea. Therefore, in addition to using camphor wood to directly cultivate A. cinnamomea, some researchers have produced camphor essential oil from other camphor laurel species, as other camphor laurel species may contain essential oil components similar to those of the stout camphor tree.
Stout camphor essential oil has a special scent due to its content of specific aromatic terpenoid compounds. Essential oils prepared from different species, varieties or subspecies have been shown to possess varied compounds[15, 16]. Of the major essential oil components extracted from the stout camphor tree, α -terpineol has been shown to be a key component, while essential oils prepared from other camphor species may not contain this terpene alcohol. Therefore, α -terpineol can be used as an index element to represent the purity of stout camphor essential oil[17, 18]. However, a method that can quantitatively examine the quality and purity of commercial stout camphor essential oils is necessary. By adding an internal standard to essential oil samples, this study aimed to develop a simple and reliable method for determining the level of α -terpineol in stout camphor essential oils on the market[19].
Fourteen stout camphor essential oil samples of different brands were purchased from markets, and stout camphor wood (C. kanehirai Hayata) was provided by a company that cultivates A. cinnamomea. α -terpineol and vanillin at a purity > 99% were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan).
Stout Camphor wood (
α -terpineol (100 mg) or vanillin (100 mg) was placed into a 100-mL volumetric flask and dissolved in isopropanol to 100 mL. The solutions used were stock solutions of α -terpineol standard (S) solution (1 000 μ g· mL-1) and vanillin internal standard (IS) solution (1 000 μ g· mL-1).
The relative response factor (
where AS is the peak area of α -terpineol and AIS is the peak area of vanillin. WS is the weight of α -terpineol, and WIS is the weight of vanillin.
The α -terpineol stock solution (1 000 μ g· mL-1) was diluted with isopropanol to concentrations of 50, 25, 10, 5, 1 and 0.5 μ g· mL-1. One mL of each diluted solution was mixed separately with 1 mL of internal standard solution (vanillin). The mixtures were injected directly into a gas chromatograph in triplicate for analysis. The coefficient of variation (CV%) for α -terpineol recovery was set at 15%; the lowest concentration of α -terpineol obtained was the lowest quantitatively-determinable concentration obtained by gas chromatography.
Twenty-five or fifty mg of each stout camphor essential oil sample were mixed with 5 mL of internal standard solution (vanillin; 1 000 μ g· mL-1). A volume of 0.1 μ L of the mixture was injected into a gas chromatograph for analysis. The recovery of each sample was measured in triplicate. The levels of α -terpineol in the essential oil samples were calculated by Equation (2):
where W is the weight of the sample.
In a 20-mL vial, α -terpineol (10 or 1 mg) was mixed with 25 mg of essential oil samples extracted from Small-flower Camphor (C. micranthum Hayat; sample S14) or stout camphor (sample S2). A blank sample was prepared without addition of α -terpineol. After adding 5 mL of internal standard solution (vanillin; 1 000 μ g· mL-1), 0.1 μ L of each mixture was injected into a gas chromatograph. The recovery level of each was measured in triplicate.
A gas chromatograph (GL Sciences 390B, Tokyo, Japan) equipped with a flame ionization detector (FID) was used with the H2 flow rate at 30 mL· min-1 and the air flow rate at 300 mL· min-1 in this study. The temperatures of the injection port and detector wese 250 and 310 ℃, respectively. The flow rate of the carrier gas (N2) was set at 5 mL· min-1. A CP-Sil 8 CB column (30 m× 0.53 mm i.d./1.0 μ m; Chrompack, Netherlands) was used.
The oven temperature was qrogrammed to initiate at 80 ℃ and hold for 3 min. The temperature was raised to 150 ℃ at a rate of 6 ℃· min-1, and hold for 1 min. Finally, the temperature was increased to 300 ℃ at a rate of 30 ℃· min-1, and hold for 10 min. The injection volume was 0.1 μ L in the direct injection mode.
We performed a test to select a suitable gas chromatography column and appropriate analytical conditions. In terms of gas chromatography column selection, a high-polar column, CP-Wax (
 | Fig.1 Gas chromatograph of α -terpineol and vanillin (IS, internal standard) authentic standard. Peak 1=α -terpineol; Peak 2=vanillin (IS) |
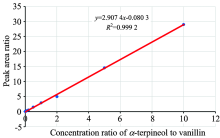
In order to accurately quantify the content of α -terpineol in the stout camphor essential oil, this study used water-soluble vanillin as an internal standard. The process first determined the RRF of α -terpineol to vanillin, and then, using the RRF value, the content of α -terpineol in each essential oil sample could be calculated according to Equation [2]. Figure 4 shows a plot of the peak area ratios (Y axis: α -terpineol/vanillin) against the concentration ratios (X axis: α -terpineol/vanillin), which demonstrates that the coefficient of determination (R2) for the linear regression model was > 0.999 and the RRF was 2.907 4 (Figure 4 and Table 1).
| Table 1 Relative response factor (RRF) and gas chromatographic retention time (RT) of α -terpineol to vanillin |
Following the addition of 50 μ L of internal standard solution (=5 μ g vanillin) to each serially-diluted standard (50, 25, 10, 5, 1 and 0.5 μ g· mL-1), the mixtures were directly injected into the gas chromatograph equipped with a FID under the conditions described in the Materials and Methods section. The result showed that when the CV% for α -terpineol recovery was set at 15%[20, 21], the lowest quantitatively-determinable concentration of α -terpineol was 5 μ g· mL-1 (Table 2).
| Table 2 Lowest quantitatively-determinable concentration of α -terpineol by gas chromatography with a flame ionization detector |
Next, we studied the recoveries of α -terpineol in two different types of essential oil samples fortified with additional α -terpineol. When 10 or 1 mg of α -terpineol was added to Small-flower Camphor essential oil (a camphor species known to contain no α -terpineol; sample S14), the recovery rates were 102.7% and 98.4%, respectively, with a CV% of 5.24% or lower (Table 3). On the other hand, when 10 or 1 mg of α -terpineol was added to stout camphor essential oil (sample S2), the recovery rates were 103.4% and 98.3%, respectively, with a CV% of 10.8% or lower (Table 4). Based on the aforementioned results, we demonstrated that our method only requires the addition of isopropanol solution that contains a known concentration of internal standard, and the mixed samples can be analyzed by gas chromatography using the direct injection mode. The analysis took only 30 min for one sample, which is fast and simple.
| Table 3 Recovery of spiked α -terpineol from small-flower camphor essential oil by the direct injection GC method |
| Table 4 Recovery of spiked α -terpineol from stout camphor tree essential oil (S2) by the direct injection GC method |
We used stout camphor essential oil prepared by ether extraction from stout camphor wood as the positive control, and determined the α -terpineol contents in the sample prepared from stout camphor wood, in commercially-available stout camphor essential oil samples, and in Small-flower Camphor essential oil (upper layer). Using the method developed in this study, the α -terpineol contents in the 14 essential oil samples were found to range from 213 to 533 mg· g-1 (Table 5). This finding indicated that the α -terpineol levels in different samples varied, and a difference of 2.5-fold was observed between the highest and the lowest contents. This is in agreement with several previous studies[20, 21, 22, 23], which reported that the components of stout camphor essential oil might vary depending on the which parts of the tree are used as the material to prepare the essential oil. In addition, the age of the tree may also affect the quality of the essential oil. This study also confirmed that essential oil extracted from the small-flower camphor tree did not contain α -terpineol.
| Table 5 α -terpineol content in some commercial Stout Camphor Tree essential oil |
In this study, a fast and simple gas chromatographic method was developed to quantitate the quality of commercially-available stout camphor essential oils. The method only required the addition of vanillin as the internal standard, and the samples can then be directly injected into a gas chromatograph to determine the level of α -terpineol, a key component of stout camphor tree essential oil. In the 14 samples we tested, the highest and lowest contents of α -terpineol were 51.6% and 21.3%, respectively. Our findings demonstrated that α -terpineol can be used as an index to rapid determine the quality of stout camphor essential oils on the market.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|