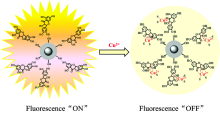
制备了一种基于天然产物槲皮素接枝硅包银核壳结构的纳米荧光传感器(Ag@SiO2@Qc), 对铜离子具有好的选择性和灵敏性。 Ag@SiO2@Qc与Cu2+离子结合后, 荧光发射强度发生猝灭, 并且可通过荧光滴定光谱得到了荧光滴定曲线: y = -32.864 x+587.59( R2=0.998), 其线性范围分别为: 3×10-7~4.8×10-6 mol·L-1, 最低检测限为1.0×10-7 mol·L-1。 并且将Ag@SiO2@Qc应用于环境中水样的检测结果的准确度好, 精密度高, 而且更加环保、 方便、 快捷, 具有很大发展潜力与应用价值。
Biography: JIANG Wei⁃na, (1986—), female, doctor in College of Chemical Engineering, Nanjing Forestry University e-mail: 121702969@qq.com
A novel fluorescent sensor based on natural quercetin and core-shell Ag@SiO2 nanoparticles for highly sensitive and selective detection of copper ions has been studied. The Ag@SiO2@Quercetin fluorescent sensor after binding to Cu2+ ions showed a quenching of fluorescence emission intensity. The sensor can be applied to the quantification of Cu2+ ions with a linear range of 3.0×10-7~4.8×10-6 mol·L-1 and a detection limit of 1.0×10-7 mol·L-1. The sensor showed high selectivity toward Cu2+. As a result, the proposed fluorescent nanosensor was successfully applied for determination of Cu2+ in water samples with good recovery.
Metal ions have a significant impact on the environment and the development of organism. Therefore, the recognition and detection of metal ions in the environment and organism is gaining widespread attention. Among the heavy metal ions, the copper ion plays a critical role in the area of environmental, biological and chemical systems[1]. Lack or excess of copper in the human body can cause growth and metabolic disorders, leading to neurological disorders such as Alzheimer’ s disease and Parkinson’ s syndrome. Also, exposure to a high level of copper can cause gastrointestinal disturbances and liver or kidney damage[2]. And high level of copper also causes pollution to the environment. Therefore, it is important to develop simple, highly sensitive and selective methods for the detection of Cu2+ ions.
New fluorescent and phosphorescent probes in which a metal-chelating group and fluorophore or phosphor exist in the same molecule have been successfully developed for the detection of cupric ions in recent years[3, 4, 5]. However, some of them have shortcomings for practical applications such as low water solubility, low fluorescence quantum yield in aqueous media and the toxicities of the ligand[6]. Many scientists are trying best to increase their application. Recent research results have demonstrated that fluorescent dye-doped nanoparticles (NPs), including quantum dots[7, 8], dye-doped silica[9, 10], gold or silver nanoparticles[11, 12] and polymer particles[13, 14, 15], have gained several advantages over conventional fluorescent sensing dyes[16], such as high brightness and improved photo stability, versatility in design and synthesis, as well as water dispersal, etc. Thus, the incorporation of fluorescent probes within nanoparticles is an attractive strategy for the application of the fluorescence technique in aqueous systems as a convenient, effective and economical chemosensor[17, 18, 19].
In this work, a kind of the silica-coated silver nanoparticles have been prepared and combined with quercetin to detect Cu2+ in water. Quercetin is a natural product widely present in most plants and belongs to flavonoid with good biological activity. It is reported that flavonoids, such as quercetin, biochanin A and isorhamnetin, could act as fluorescent sensors for detecting copper ions[20]. Moreover, they are eco-friendly. The majority of the fluorescent sensors are synthesized by organic reaction with toxic reagent, and only few fluorescent sensors come from isolated phytochemicals. Quercetin is one of the effective metal chelators which possess three possible chelating sites in competition: the 3-hydroxy-4-carbonyl, the 5-hydroxy-4-carbonyl, and the 3’ , 4’ -dihydroxy (catechol). Complexation of metal cations may change its spectral characteristics. Thus quercetin has huge potential for detection of metal ions based on the changes of its fluorescence intensity. However, the fluorescence intensity of quercetin is lower, which would limit application. Herein, a facile route was proposed for preparation of a new nanoparticle for detection of Cu2+ by modifying quercetin on the silica-coated silver nanoparticles (denoted as Ag@SiO2@Quercetin), showing not only strong fluorescence intensity and lower detection limit but also high selectivity and sensitivity to Cu2+.
The following chemicals were used for preparation of core-shell Ag@SiO2 nanocomposites: silver nitrate (AgNO3, AR), sodium citrate (Na-cit, AR), tetraethylorthosilicate (TEOS, AR), sodium benzoate (C6H5COONa, AR), anhydrous ethanol (EtOH, AR), ammonia (NH3· H2O, AR).
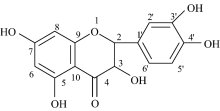
Dried Flos Sophorae powder (18 g) were exhaustively extracted according to relevant paper[20]. The product was recrystallized from ethanol to afford yellow crystals (1.018 2 g, yield 6.0%). M. p.> 300 C. 1H NMR (DMSO-d6, 600 MHz): 12.50 (s, 1H, 5-OH), 10.78 (s, 1H, 7-OH), 9.59 (s, 1H, 3-OH), 9.37 (s, 1H, 4’ -OH), 9.31 (s, 1H, 3’ -OH), 7.68 (d, J=1.8 Hz, 1H, 2’ -H), 7.54 (d× d, J1
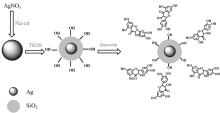
The Ag@SiO2 was prepared by slightly improved method according to literature[21]. The synthetic route for Ag@SiO2 is presented in Scheme 2.
After the mixture of 2 mL 0.129 mol· L-1 AgNO3, 1 mL 0.200 mol· L-1 sodium citrate and 250 mL redistilled water was stirred vigorously and refluxed for 20 min. It was centrifuged for 0.5 h at 3 000 r· min-1 to remove the larger silver nanoparticles and then obtained silver colloid.
In order to synthesize the Ag@SiO2 nanoparticles, 10 mL silver colloid, 30 mL anhydrous ethanol, and 1 mL sodium citrate solution were stirred vigorously under pH 8. Then TEOS were added, and the reaction was maintained for 20 h.
In order to synthesize Ag@SiO2@Quercetin, 3.00 mL 1.0× 10-5 mol· L-1 of Quercetin was added into 0.1 mL Ag@SiO2 solution. The mixture was stirred for 1 h and then allowed to stand for another 10 min to equilibrium, after which the fluorescence spectra were detected.
Transmission electron microscopy (TEM, JEM-1400) was used to investigate themorphology and structure of the core-shell Ag@SiO2 nanoparticles. The particle size of Ag@SiO2 was investigated by means of laser particle analyzer (Mastersizer 2000). Fluorescence spectra were recorded with a Perkin-Elmer LS55 spectrophotometer, and the excitation wavelength was 390 nm for all measurements. The content of copper ions were measured on AA900T AAS
In order to check the fluorescence response of Ag@SiO2@Quercetin to different metal ions, stock solutions(1× 10-3 mol· L-1) of acetate/nitrate salts of different metal ions (Pb2+, Zn2+, Co2+, Tb3+, Nd3+, Ag+, K+, Sb3+, Cd2+, Mn2+, Hg2+, Ni2+, Fe3+, Fe2+, Ho3+, Cu2+). The test solutions were prepared by 3 mL of the sensor stock solutions and a small quantity of certain ion stock solutions. In the test solutions, [Cu2+]=1.0× 10-5 mol· L-1, the concentration of other metal cations was 1.0× 10-5 mol· L-1.
In order to check the influence of other metal ions on fluorescence responses of Ag@SiO2@Quercetin to Cu2+, test solutions were prepared by placing 3 mL of the sensor stock solution and 5μ L of Cu2+ stock solution into a test tube, then adding a small quantity of certain metal ions stock solution to it. In the test solutions, [Cu2+] = 1× 10-5mol· L-1and the concentration of other metal cations was 1× 10-4 mol· L-1.
We designed an experiment to detect Cu2+ in aqueous solution using Ag@SiO2@Quercetin as fluorescence sensor. Water samples were collected from suburban and urban areas in Nanjing and Taizhou. Water samples were filtrated through a 0.22-μ m-pore-size membrane. Nitric acid digestion was performed before metal analysis by ASS and Ag@SiO2@Quercetin fluorescent sensor.
TEM images of silver nanoparticles and Ag@SiO2 nanoparticles are shown in Fig.1. Fig.1(a) showed that most of the silver nanoparticles are nearly spherical and their average diameter is about 40 nm. Fig.1(b) exhibits that the silver nanoparticles are coated with SiO2 shell. XPS results suggest there are Ag and Si elements in Ag@SiO2 nanoparticles (Table 1).
| Table 1 X-Ray Photoelectron-Spectroscopy of Ag@SiO2 nanoparticles |
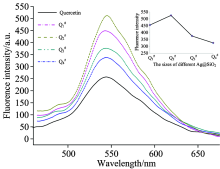
Because the concentration of TEOS will affect the rate of hydrolysis and polymerization, the SiO2 shell thickness of the corn-shell Ag@SiO2 could be controlled by the concenuration of TEOS. Fig.2 shows the sizes of four kinds of core-shell Ag@SiO2 nanoparticles via controlling the concentration of TEOS on 40 nm silver core. And sizes of 1#, 2#, 3# and 4# are 50, 60, 95 and 120 nm, respectively, in which the SiO2 shell thickness of 1#, 2#, 3# and 4# are 10, 20, 55, 80 nm respectively (shown in Table 2).
| Table 2 The sizes of Ag@SiO2 nanoparticles and the thickness of SiO2 shell of 1#, 2#, 3#, 4# |
Ag@SiO2@Quercetin Q1#, Q2#, Q3#, Q4# was obtained by adding Ag@SiO2 1#, 2#, 3#, 4# into Quercetin solution, respectively. The fluorescence intensities of Q1#, Q2#, Q3#, Q4# was investigated, shown in Fig.3. The results indicated that Ag@SiO2@Quercetin nanocomposites with different SiO2 shell thickness all showed stronger fluorescence intensities than quercetin, and the emission intensity first increased then decreased with the increase of the thickness of SiO2 shell. When the thickness of SiO2 shell was 10 nm (in Q1#), the distance between quercetin and Ag core was too close that it may lead to non-radiative energy transfer between quercetin and Ag@SiO2 nanoparticles. While the thickness of the SiO2 shell was 80 nm (in Q4#), the distance between the quercetin and Ag nanoparticles was too far and the electromagnetic field around the quercetin was weak, so the emission intensity showed weaker than others. In Q2#, the SiO2 shell was 20nm, which can not only avoid molecular fluorescence quench but also make sure that the electromagnetic field around the quercetin was stronger[22, 23, 24, 25], so Q2# showed the strongest emission intensities. Therefore, Ag@SiO2@Quercetin Q2# was investigated as sensor in the detection of copper ions in later assay.
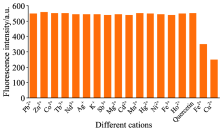
To evaluate the selectivity of the Ag@SiO2@Quercertin system, many metal ions (Pb2+, Zn2+, Co2+, Tb3+, Nd3+, Ag+, K+, Sb3+, Mg2+, Cd2+, Mn2+, Hg2+, Ni2+, Fe3+, Ho3+, Cu2+) were used for the nanosensor study. Each of the ions was added to the prepared detection system individually, and the fluorescence intensity variation of Ag@SiO2@Quercertin was psesented in Fig.4, which indicated that only Cu2+ and Fe2+ induced obvious fluorescence quenching at their corresponding peaks (546.5 nm). However, Fe2+ is not stable and oxidizes to Fe3+ easily under natural conditions, which cannot affect the sensing process of Cu2+ in lakes and rivers. So, Fe2+is not involved in the discussion. And none of the other metal ions (Pb2+, Zn2+, Co2+, Tb3+, Nd3+, Ag+, K+, Sb3+, Mg2+, Cd2+, Mn2+, Hg2+, Ni2+, Fe3+, Ho3+)generated a significant influence on fluorescence signals. Thus, the sensing process can happened in the presence of Cu2+ with fluorescence quenching.
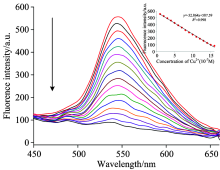
For Cu2+ detection, 3 mL of the as-prepared fluorescent nanosensor (Q2#) was added into the spectrophotometer quartz cuvette, and the emission spectra of the nanosensor were recorded from 450 to 650 nm. Then 0.3, 0.6, 1.0, 1.4, 1.8, 2.2, 2.6, 3.0, 3.4, 3.8, 4.0, 4.2, 4.4, 4.6, 4.8 μ mol· L-1 of Cu2+ solution were added into the sensor solution, and the fluorescence intensity (546.5 nm) of Q2# gradually decreased as the Cu2+ concentrations increased (Fig.5). The process of fluorescence quenching was mostly related to static quenching that originated from the formation of non-fluorescent Ag@SiO2@Quercetin-Cu(Ⅱ ) complex[13].
The fluorescence titration experiments showed that the fluorescence intensity was inversely proportional to copper ion concentrations, and gave a good linear change in fluorescence emission intensity in response to the concentration of Cu2+ ranging from 3.0× 10-7 to 4.8× 10-6 mol· L-1. The calibration curve was y=-32.864x+587.59(R2=0.998). The detection limit of Cu2+ was as low as 1.0× 10-7 mol· L-1. The standard curve with good linearity can be used for quantification.
Furthermore, fluorescence responses of Ag@SiO2@Quercetin to Cu2+ in the presence of certain other metal ions were also tested (shown in Fig.6), and the result showed that these competitive cations did not lead to any significant change of fluorescence intensities even in a high concentration. Therefore, the presence of other metal ions hardly interfered with the fluorescence response of the Ag@SiO2@Quercetin-Cu(Ⅱ ) system, so Ag@SiO2@Quercetin showed high selectivity for sensing of Cu2+.
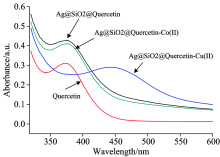
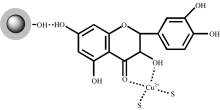
We speculate that the fluorescence quenching sensing mechanism could result from the intramolecular charge transfer (ICT) according to previously reported paper[20]. The UV-visible spectra of Ag@SiO2@Quercetin in the presence of Cu2+ and Co2+have been depicted in Fig.7. It clearly expresses that there is an obviously change in the characteristics of the Ag@SiO2@Quercetin when addition of Cu2+ into the Ag@SiO2@Quercetin solution while there is no change in addition of Co2+. The UV-visible spectrum of Ag@SiO2@Quercetin in buffer solution shows an absorption band at 375 nm which are related to the π — π * transitions within the aromatic rings, similar to Quercetin solution. It also shows that the absorbance of Ag@SiO2@Quercetin is stronger than quercetin in the same concentration. Then the addition of Cu2+ into Ag@SiO2@Quercetin solution shows a distinguishable change in the absorption band with shifting to the long-wavelength region (430 nm). Such bathochromic shift can be explained by the extension of the conjugated system with the complexation. Scheme 3 shows the most probable structure of Ag@SiO2@Quercetin-Cu(Ⅱ ) complex. As the 3-hydroxy group of quercetin has a more acidic proton, therefore the 3-OH and 4-carbonyl groups are the most probable coordination sites to be involved in the complexation process between Ag@SiO2@Quercetin and Cu2+ which leads to a change of the UV absorption band. When Cu2+ reacted with Ag@SiO2@Quercetin system, Ag@SiO2@Quercetin-Cu(Ⅱ ) complex obtained, and intramolecular charge transfer (ICT) took place due to the extension of the conjugated system, subsequently, fluorescence quenching of Ag@SiO2@Quercetin system occurred (scheme 4).
To demonstrate the feasibility of the sensor Ag@SiO2@Quercetin, we applied this method for detecting Cu2+ in several rivers and lakes with Ag@SiO2@Quercetin as fluorescent sensor. The results were checked by ASS (shown in Table 3). Compared with ASS method, the results obtained by the sensor were also accurate and had good recovery, which proved that Cu2+ can be detected quantitatively by using Ag@SiO2@Quercetin as sensor with this method.
| Table 3 Determination of Cu2+ ions in water samples using the Ag@SiO2@Quercetin fluorescent sensor |
In conclusion, a novel fluorescent sensor based on natural product and core-shell nanoparticles was prepared via a facile synthetic strategy. This work will opens up new possibilities for Quercetin as a chemosensor in quantification, and suggests potential applications of Quercetin not only in biochemistry but also in environmental research. Upon grafting of a natural product, the Ag@SiO2@Quercetin nanoparticle can serve as a new fluorescence sensor that responds sensitively and selectively to Cu2+ in aqueous media. The result also suggests that such highly sensitive and selective materials for sensing ions in aqueous solution will have potential as luminescent sensors in biological systems in the future.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|