采用微波加热和常规电加热两种条件进行液化残渣(DCLR)的热解实验, 考察了热解产物固体焦、 焦油及煤气的组成及结构的变化规律, 采用红外分析(FTIR)与气相色谱-质谱(GC-MS)联用技术对热解产品进行了分析表征。 研究表明, 在微波场中, DCLR的升温速率很快, 20 min左右物料温度就可达到900 ℃, 最大升温速率可达到329 ℃·min-1, 而常规加热的升温速率基本保持恒定。 与常规热解相比, 微波热解后固体焦的产率降低2.85%, 而焦油和煤气产率分别增加了0.66%和2.19%。 DCLR热解后固体焦的索氏萃取组分重油(HS)、 沥青烯(A)及前沥青烯(PA)含量均大幅降低, 而四氢呋喃不溶物(THFIS)则有所增加, 但是两种热解条件下得到的固体焦的四种索氏组成差异不是很大, 说明DCLR的热解过程是以HS, A与PA的转化为主的。 微波热解后固体焦红外谱上3 437.6, 1 632.0 cm-1以及1 079.99 cm-1处吸收峰的强度与常规热解相比明显降低, 说明微波场中DCLR的热解更为彻底。 热解后焦油和煤气产率均有所增加, 煤气中H2含量均达到60%以上。 GC-MS分析表明, 经由石油醚萃取后的热解焦油中脂肪类、 芳香类与醇类物质组成以及C1~5, C11~20与C20以上组分的含量均没有发生明显变化, 而微波热解焦油中沥青质的含量则下降了7.7%, 说明微波作用可有效促进DCLR中沥青质的热分解, 有利于热解焦油的轻质化。
The pyrolysis of direct-coal-liquefaction residue (DCLR) was prepared using microwave and conventional pyrolysis. The composition and structure of solid coke, tar and gas were investigated and the pyrolysis products were compared after characterization by infrared spectroscopy (FTIR) and gas chromatography-mass spectrometry (GC-MS).Results showed that DCLR was rapidly heated up to 900 ℃ in 20 min with the maximum heating rate of 329 ℃·min-1 in the microwave field, while the heating rate of conventional heating was constant. Compared with conventional pyrolysis,coke yield decreased by 3% after microwave pyrolysis, whereas the tar and gas yields increased by 0.66% and 2.19%, respectively.After the pyrolysis of DCLR, the extracted compositions consisting of heavy oil(HS), as phaltene (A), and pre-asphaltene (PA) decreased significantly, while the content of tetrahydrofuran insolubles (THFIS) increased. The Soxhlet compositions of the solid coke did not evidently change compared with those after conventional pyrolysis, which indicated that the pyrolysis process of DCLR is mainly based on the conversion of HS, A and PA. After microwave pyrolysis,the intensity of the absorption peak at 3 437.6, 1 632 and 1 079.99 cm-1 on the infrared spectrum of the solid coke was significantly lower than that of conventional pyrolysis, which indicated that DCLR was more thoroughly pyrolyzed in the microwave field.Both tar and gas yields increased after pyrolysis, and the content of H2 in gas reached above 60%. The results of GC-MS showed that no obvious change in the composition for aliphatics, aromatics, alcohol and the contents of C1~5, C11~20 and C>20in tar was observed after extraction with petroleum ether. The asphaltene content of tar decreased by 7.7% after microwave pyrolysis, which indicated that microwave pyrolysis can effectively promote asphaltene decomposition in DCLR, which benefited tar conversion to light fraction.
Introduction
Direct-coal-liquefaction residue (DCLR) is a hydrogen-rich substance consisting of about 30% heavy oil and 25% asphaltene and pre-asphaltene with high sulfur, carbon, and ash content[1]. Oil recovery from the pyrolysis or hydro-pyrolysis of DCLR is one of the simplest, most economical, and most reliable methods of comprehensively utilizing DCLR[2, 3, 4]. Zhu Yufei[5] studied the pyrolysis of direct-coal-liquefaction residue at 550 ℃ for 2 h, and found that when the temperature of pyrolysis increased, the yield of heavy distillate in pyrolysis oil (> 450 ℃) increased, indicating that if the temperature of pyrolysis was higher, the polymerization rate of oil vapor produced by pyrolysis of residue at high temperature was faster. In the study of co-pyrolysis of direct-coal-liquefaction residue and lignite, Li Xiaohong[6] found that there was interaction in co-pyrolysis process between residue and lignite. The addition of residue could provide hydrogen for the pyrolysis process, but residue also had a mass transfer barrier in the co-pyrolysis process. The experimental results showed that when the pyrolysis temperature was 550 ℃ and the mass ratio of residue and lignite was less than 0.15∶ 1, the tar yield was higher than the theoretical value. Li Xiaohong[7] studied the properties of semi-coke from co-pyrolysis of lignite and direct-coal-liquefaction residue of Shendong coal, and found the specific surface area and pore volume of co-pyrolysis semi-coke decrease, while the order degree of co-pyrolysis semi-coke increases with the addition of DCLR. In the meanwhile, compared with the lignite semi-coke, the CO2gasification reactivity of co-pyrolysis semi-coke decreases. LI Jun et al.[8, 9] found that the hydrotreatment of heavy liquid in DCLR can improve oil yield. Moreover, they showed that the interaction of extracts in DCLR resulting in certain low-temperature volatile components does not escape the low-temperature region and decomposes in the high-temperature region, thus meeting the requirement of hydrogenation liquefaction of radical fragments.
In the dry, desulfuration and pyrolysis of coal and oil shale, microwave application is eliciting profound attention because of this method’ s high heating rate, uniform heating, and energy efficiency[10]. T. Uslu[11] showed that coal temperature quickly increases to > 1 000 ℃ in 3 min in a microwave field when absorbing materials such as copper oxide and ferroferric oxide existed. The technology for coke production from bituminous coal with high volatility using microwave energy has been studied by Ed Lester et al.[12] and found that this coal-pyrolysis method is effective. SONG et al.[13]experimentally demonstrated that low-rank coal is rapidly pyrolyzed in a microwave field, and that tar yield through microwave pyrolysis increases by 3%~5% compared with tar yield through conventional pyrolysis. Moreover, the light fraction content of tar increases, and the proportion of H2 in gas reaches about 50%.
This study compared microwave and conventional pyrolysis methods, investigated heating characteristics and compositions, and examined the variation pattern of pyrolysis products for DCLR. Our results can lay a foundation for rapid-pyrolysis technology by using microwave energy for DCLR.
DCLR from the direct liquefaction product of the Shenhua Group was used as a sample, whose proximate and ultimate analyses are shown in Table 1. Results suggested that DCLR contained highly volatile matter, carbon, and ash content.
| Table 1 Proximate and ultimate analyses of DCLR (%) |
Microwave and conventional pyrolysis methods were carried out in a MKX-J2B microwave oven and a GXL1400X vacuum-tube furnace respectively. About 100 g of DCLR was added to a quartz reactor, which was then placed inside the furnace. After eliminating the entrapped air in the reactor by using an inert gas, the reactor was heated and maintained at the desired power (800 W) or temperature (900 ℃) for 40 min. Tar was collected as the pyrolysis gas passed through the two-stage condensation system with a cooling medium temperature of 0 ℃, and then the gas was analyzed using an online gas analysis meter after drying. The power was turned off, and the coke samples were collected for subsequent analysis after naturally cooling at room temperature.
Four-component analyses of DCLR and coke were carried out according to the national standard of coking products. Toluene-insoluble content was determined in a Soxhlet apparatus with an Allihn condenser. Gas composition was analyzed using a Gasboard-3100 online infrared analyzer. The structure and composition of coke and tar were characterized using an IR Prestige-21 Fourier transform infrared (FTIR) spectrometer and a gas chromatography (6890)-mass spectrometry (5973) system respectively.
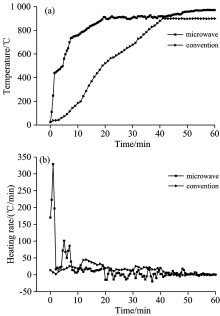
As shown in Fig.1, DCLR was rapidly pyrolyzed up to 700 ℃ in 8 min in a microwave field with the maximum heating rate of 329 ℃· min-1, which was then slightly decreased, although 900 ℃ was still reached in 20 min. No obvious change in temperature was observed afterwards. By contrast, the temperature of DCLR in a conventional field reached 900 ℃ in about 45 min at an almost constant heating rate. Microwave heating is a kind of volumetric heating induced by the dielectric loss of a material in an electromagnetic field. This heating process is closely related to internal molecular polarization. Heat transfers from an internal to an external surface with high transfer efficiency and heating rate. Moreover, DCLR contains plenty of pyrrhotite, which has excellent microwave absorbing properties and can reach 955 K in 42s. Moreover, DCLR could form “ hot spots” during pyrolysis, resulting in increased heating rate.
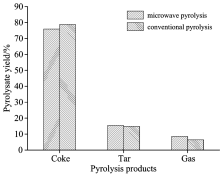
As shown in Fig.2, coke obtained after DCLR pyrolysis in a microwave field decreased by 2.85% compared with that in a conventional field, whereas tar and gas yields increased by 0.66% and 2.19% respectively. Material temperature can reach 900 ℃ in 40 min regardless of heating method (Fig.1). Meanwhile, abundant volatiles were released accompanying DCLR pyrolysis. The increased heating rate plays a significant role in improving DCLR pyrolysis product yield.
The heating rate of microwave pyrolysis was faster than that of conventional pyrolysis. Consequently, the produced gas products rapidly released, resulting in more thorough volatile evolution, decreased solid product yield, and increased gas product yield.
As shown in Table 2, gas compositions roughly had the same proportion under conventional and microwave pyrolysis conditions, except for H2 content, which increased by 3.61% in a microwave field. Temperature variations can significantly affect pyrolysis gas composition and yield according to the results shown in Fig.1, in which the terminal pyrolysis temperature reached 900 ℃ under both pyrolysis conditions. Consequently, the gas compositions aligned well. DCLR is a hydrogen-rich substance, and H radicals were mainly the result of the poly-condensation reaction of the aromatic compounds during pyrolysis, especially the hydrogenated aromatic compounds that were involved in condensation, dehydrogenation, and poly-condensation reactions. During the microwave field, the fast pyrolysis promoted the cracking of dehydrogenation reaction and increased the content of hydrogen gases such as H2, CH4, CnHm.
| Table 2 Gas compositions under different heating methods (V/%) |
| Table 3 Contents of char and DCLR compositions under different heating methods (%) |
Results in Table 3 suggested that asphaltene, pre-asphaltene and most of the heavy oil were pyrolyzed during microwave and conventional pyrolysis, in which heavy oil content decreased from 26.01% to 6.70% (microwave pyrolysis) and 7.95% (conventional pyrolysis), respectively. Preasphaltene was completely pyrolyzed, and asphaltene content decreased from 30.08% in DCLR to 0.19% in the coke, whereas the tetrahydrofuran-iosoluble content of coke was still > 90%, indicating that recovering oil first through pyrolysis was a reasonable way to upgrade DCLR.
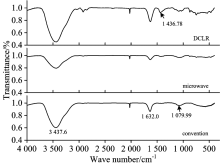
The FTIR spectra of DCLR and coke are shown in Fig.3. The broad and intense characteristic absorption peak near 3 437.6 cm-1 was assigned to the — OH functional group and used to measure alcohol or phenol content existing in the form of dimer or polymer. The bands between 3 000 and 2 500 cm-1 were assigned to saturated — C— H stretching vibration mode. The bands near 1 632.0, 1 436.78, and 1 079.99 cm-1 were assigned to — C=C— functional group, saturated and unsaturated — C— H bending vibration mode, and — C— O stretching vibration mode, respectively. The bands between 1 300 and 300 cm-1 were fingerprint region attributed to — C— X bending and stretching vibration and decomposition of inorganic mineral substance. As is shown in Fig.3, the intensities of absorption peaks near 3 437.6, 1 632.0, and 1 079.99 cm-1 significantly decreased, whereas the absorption peaks that varied from 3 000 to 2 500 cm-1 and near 1 436.78 cm-1 almost disappeared during microwave and conventional pyrolysis, implying that all corresponding functional groups were involved. The intensities of the absorption peaks at 3 437.6, 1 632.0, and 1 079.99 cm-1 in the FTIR spectra obtained under microwave pyrolysis condition were lower than that under conventional condition, indicating that DCLR was more thoroughly pyrolyzed in the microwave field.
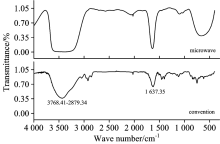
Fig.4 shows the FTIR spectra of tar obtained microwave and conventional pyrolysis methods. The bands between 3 768.41 and 2 879.34 cm-1 were assigned to — OH, unsaturated C— H, and saturated — C— H stretching vibration mode. The absorption peak near 1 637.35 cm-1 was assigned to aromatic — C=C— , whereas the bands from 1 300 to 300 cm-1 were assigned to — C— X stretching and bending vibration. The intensities of absorption peaks in the FTIR spectra obtained under microwave pyrolysis condition were higher than those under conventional condition. The wide absorption peaks between 3 768.41 and 2 879.34 cm-1 were attributed to the interference of characteristic vibrations with the close wavelength and vibration coupling of certain absorbance, produced double peaks that looked like a widened mono-peak at low resolution. This result suggested that tar contained plenty of hydroxyl groups, as well as saturated — C— H and unsaturated C— H groups. The heating rate in the conventional pyrolysis was low, hence the relatively long residence time for tar. This long residence time prompted the secondary cracking of tar that led to decreased intensity of the corresponding absorption peaks. These results suggested that the content of unsaturated functional groups in the tar can be efficiently improved under optimum pyrolysis conditions in microwave and conventional fields, thereby improving the utilization value of tar.
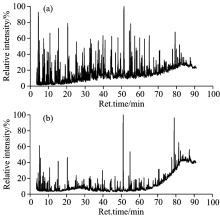
Only organic compounds with < 300 ℃ boiling point can be detected using GC/MS. Thus, tar was first extracted using petroleum ether before analysis, and insoluble substances after extraction were mainly asphaltene. Results of extraction experiments showed that the extraction rates of tar obtained by microwave and conventional pyrolysis methods were 69.7% and 62.0% respectively, indicating that the asphaltene of tar obtained by microwave pyrolysis was lower than that by conventional pyrolysis. Data in Tables 4 and 5 shows that the contents of fatty hydrocarbons, aromatic hydrocarbons, and phenols in tar after extraction were approximately similar, as well as the contents of C1~5, C11~20, and above C20 in the tar obtained under the two pyrolysis conditions. The petroleum extraction rate of microwave pyrolysis products was 69.7%, which was higher than that of conventional pyrolysis products, indicating a higher light fraction in tar after microwave pyrolysis. In addition, the GC/MS chromatograms of tar in Fig.5, where the horizontal ordinate representing the mass-to-charge ratio, depicted that quasi-molecular ions were positioned forward and intensity increased under microwave heating method. It was suggested that the light fraction accounted for a high proportion of tar and microwave pyrolysis contributed to tar conversion to light fraction. This was because some of the molecules thermally decomposed (thermal effect), and another part vibrated to break the molecular chains (non-thermal effect) in the microwave field, there by prompting the decomposition of most of heavy oil and asphaltene. Asphaltene and polycyclic aromatic hydrocarbons in DCLR absorbing microwaves can reduce heavy oil and asphaltene system viscosity. With increased system temperature, the internal energy of molecules increased until it was higher than the C— C and C— H bond energy, and then the organic molecules bonds broke. Consequently, the absorption microwave ability, rate, and efficiency of DCLR pyrolysis improved.
| Table 4 Tar compositions under different heating methods (%) |
| Table 5 Distribution of different carbon numbers in tarunder different heating methods (%) |
DCLR was rapidly pyrolyzed in a microwave field with high tar and gas yields. The microwave effect can enhance asphaltene thermal decomposition, which contributed to tar conversion to light fraction. The maximum heating rate of DCLR can reach up to 329 ℃· min-1 in the microwave field. Coke yield decreased after microwave pyrolysis, where as tar and gas yields increased. The Soxhlet compositions consisting of heavy oil, asphaltene, and pre-asphaltene in the tar had no evident change compared with that after conventional pyrolysis. The content of hydrogen gases such as H2, CH4, CnHm increased within the microwave field. No obvious change in the composition of aliphatics, aromatics, and alcohol was observed in tar after extraction using petroleum ether, whereas the asphaltenes content of tar decreased by 7.7%.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|