在模拟人体生理条件下, 应用光谱法和分子对接技术对氟罗沙星(FIE)与溶菌酶(LYSO)的相互作用进行了研究。 结果表明, FIE与LYSO的猝灭方式是静态猝灭, 且在298和310 K温度下的猝灭常数 Ka分别为4.10×104和0.74×104 L·mol-1。 根据热力学参数的计算结果可知, FIE与LYSO的结合作用力主要是氢键和范德华力, 结合距离( r=3.16 nm, <8 nm)表明从LYSO到FIE发生了非辐射能量转移。 希尔系数的计算结果表明, 在不同温度下的 nH<1, LYSO和FIE的相互作用属于负协同作用。 圆二色谱结果表明, LYSO和FIE的结合使LYSO的α-螺旋的含量由21.1% 减少到 8.8%。 紫外光谱、 三维荧光光谱、 同步荧光光谱结果表明, LYSO和FIE的相互作用改变了LYSO的构象和微环境。 分子对接进一步显示FIE通过氢键、 极性键、 疏水作用力等与LYSO活性部位的ASP-52, TRP-62, TRP-63等氨基酸残基相互作用。 溶菌酶的活性实验表明, 由于以上实验结果说明FIE引起了LYSO的构象改变, LYSO的活性随着FIE浓度的增大而降低, 抑制了LYSO的活性。 该研究结果为阐明FIE在机体内与LYSO的结合机理提供了可靠的实验数据和结果, 为FIE对溶菌酶的的毒性评价和毒理学研究提供了理论依据。
The interaction of fleroxacin (FLE) with extracelluar protein (lysozyme, LYSO) was investigated by using multi-spectral techniques and molecular docking in vitro. Fluorescence spectra studies indicated that FLE quenched LYSO fluorescence in a static mode with binding constants ( Ka) of 4.10×104 and 0.74×104 L·mol-1 at 298 and 310 K, respectively. The thermodynamic parameters demonstrated that hydrogen bonds and van der Waals forces played the major role in the binding process. Based on the Förster theory of nonradiative energy transfer, the binding distance ( r) between FLE and the inner tryptophan residues of LYSO was calculated to be 3.16 nm. The Hill’s coefficient ( nH) was calculated by using involved equations, implying the negative cooperativity between them. Circular dichroism spectra (CD) indicated the secondary structure of LYSO was partially destroyed by FLE with the α-helix percentage decreasing from 21.1% to 8.8%. UV-Vis spectral, synchronous fluorescence and three-dimensional fluorescence spectra revealed the binding interaction could cause conformational and micro-environmental changes of LYSO. The results of molecular docking showed that FLE was mainly bound in the active site hinge region where ASP-52, TRP-62 and TRP-63 were located, and well supported the thermodynamic results. Besides, FIE made the activity of lysozyme decrease with the increasing concentration of fleroxacin, indicating that structural changes in lysozyme can cause the inhibition of lysozyme activity. The work clarified the interaction mechanism of FLE with LYSO at molecular level.
Introduction
Fleroxacin (FLE, the chemical structure is embedded in Fig.2), is a synthetic fluoroquinolones antibacterial of the third generation widely used in the treatment of urinary infections, and was exceedingly used in human and veterinary medicine because of its excellent activity against both gram-positive and gram-negative organisms and inhibiting the bacterial enzyme DNA gyrase, entering the environment mainly via feces and urine[1]. The residual FLE have found in milk, eggs, meats, surface and ground water, and even aquaculture, which will give rise to public health concerns, including development of resistant bacterial strains, allergic hypersensitivity reactions, and even toxic effects[2].
Lysozyme (LYSO), which is a model protein as extracellular protein, not only distributes in almost all the secretions and tissues in human body[3], but protects higher organisms from the infection of microorganisms because of its physiological and pharmaceutical functions such as antiseptic, antioxidant, antiviral, and antineoplastic effects[4]. Generally, intake of any contaminants is likely to affect the activity of the enzyme in vivo[4].
Up to now, many studies were mainly carried out on the interactions between environmental contaminants and human serum albumin (HSA), and different quinolones antibacterial ligands were also reported to bind with HSA. However, as a emerging contaminant, whether and how FLE affect the structure and function of LYSO still remains unclear. The mechanism of the FLE to LYSO is helpful for understanding possible delivery and distribution of the FLE in vivo, and illustrates FLE potential toxicity to cause structural damage of LYSO, suggesting higher potencies in causing adverse effects on human health.
In this paper, the interaction between FLE and LYSO was thoroughly investigated by fluorescence spectra, UV-Vis absorption spectra, synchronous fluorescence spectra, circular dichroism, and molecular docking techniques under simulative physiological conditions. The obtained results are beneficial to the understanding of the distribution and transport of FLE in the human body and the subsequent interactions with targeted receptor, and provide essential information for the safe use and risk assessment of FLE.
FLE (National Institutes for Food and Drug Control, Beijing, China) was dissolved and diluted to 1.0× 10-3 mol· L-1 with ultrapure water. 5.0× 10-5 mol· L-1 LYSO (Sigma, Missouri, USA) work solutions were prepared. Phosphate buffer solution and 1.0 mol· L-1 NaCl solution were used. Lysozyme kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China).
Fluorescence spectra, UV-Vis absorption spectra, synchronous fluorescence spectra, circular dichroism, and molecular docking were finished according to Ref.[5]. The activity of lysozyme was measured by the conventional turbidimetric method according to Ref.[6].
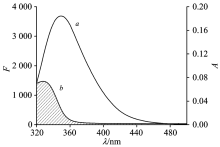
Fig.1 showed the effect of increasing concentration of FLE on the fluorescence emission spectrum of lysozyme. It can be seen that the LYSO can be quenched by FLE, which indicating that the interaction occurred and the non-fluorescent complex formed between FLE and LYSO. In addition, an obvious red shift of the maximum emission peak was found in the LYSO emission spectra when the FLE concentration was increased, indicated that the binding of FLE with LYSO caused the quenching of LYSO intrinsic fluorescence and the microenvironmental changes around the fluorophores[7]. In order to eliminate the inner filter effects and confirm the quenching mechanism, the Stern-Volmer equation[5] was used for calculating fluorescence quenching data.
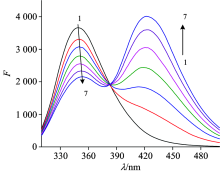 | Fig.1 Fluorescence spectra of LYSO in the presence of FLE at 298 K cLYSO=6.0× 10-6 mol· L-1; cFLE(× 10-6 mol· L-1) (1→ 7): 0.0; 1.6; 3.2; 4.8; 6.4; 8.0; 9.6 |
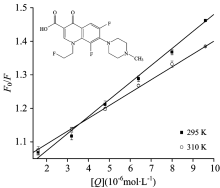 | Fig.2 Stern-Volmer curves for the binding of FLE to LYSO at 298 and 310 K, respectively cLYSO=6.0× 10-6 mol· L-1 |
The corrected Stern-Volmer plots for the quenching of LYSO by FLE at different temperature were shown in Fig.2. The calculated Ksv and kq values were listed in Table 1. There was a good linear dependence between F0/F and [Q], and the values of Ksv deceased and kq values were all greater than the upper limit of 2.0× 1010 L· mol-1· s-1, revealing the occurrence of static quenching interaction between FLE and LYSO.
FLE-induced fluorescence quenching of LYSO was a static process, and the binding constant (Ka) and the binding sites (n) can be calculated according to Ref.[5]. It can be shown in Table 2, Ka values decreased indicated that there is a strong interaction and high temperature reduces the stability of FLE-LYSO complex[7]. The number of binding sites n about equaled to 1, which revealed that the LYSO-FIE compound had one bingding site.
| Table 1 Stern-Volmer quenching constants of FLE-LYSO system |
| Table 2 Thermodynamic parameters and Hill’ s coefficients of FLE-LYSO system |
The enthalpy change (Δ H), entropy change (Δ S) and free energy change (Δ G) were evaluated by using Van’ t Hoff equation according to Ref.[5].
In Table 2, the Δ G values were negative, indicating the spontaneity of the binding of FLE with LYSO. And the negative Δ H and negative Δ S suggested that van der Waals forces and hydrogen bonds had major functions during the interaction of FLE and LYSO.
The Hill’ s equation was used to estimate the cooperativity in multisubunits’ allosteric proteins. Hill’ s coefficient was calculated graphically on the basis of the equation of Ref.[8]. If the nH value is smaller than 1 (nH< 1), it confirms the negative cooperative binding, and vice versa[8]. The calculated nH values at different temperatures were listed in Table 2. The values of nH were smaller than 1, which indicated that the negative cooperativity may play a role in the binding of FLE complex with LYSO.
Fö ster’ s non-radiative energy transfer theory can determined the values of the distance (r) between the acceptor and donor and the critical energy transfer distance (R0). J can be evaluated by integrating the spectra in Fig.4. For LYSO, K2=2/3, n=1.36, and Φ =0.14[9], and the following parameters were obtained: J=1.70× 10-14 cm3· L· mol-1, R0=2.72 nm, E=0.29 and r=3.16 nm. If r< 8 nm, which impled that energy transfer had occurred from LYSO to FIE[9]. Besides, the results were in line with Fö ster’ s non-radiative energy transfer theory and well supported the quenching results between LYSO and FIE.
It can been seen that there was a strong absorption peak at the wavelength of 202 nm and a shoulder peak at 278 nm in Fig.3, which reflected the framework conformation of the LYSO and amino acid residues, respectively[4]. The decrease of the peaks at 202 and 278 nm, and red shifts (the figure was embedded in Fig.3) indicated that the interaction between FLE and LYSO changed the protein skeleton and microenvironment of lysozyme.
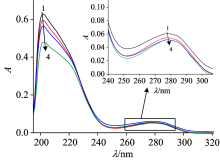 | Fig.3 UV-Vis spectra of LYSO-FLE system at 298 K cLYSO=2.0× 10-6 mol· L-1; cFLE(× 10-5 mol· L-1)(1→ 4): 0; 0.8; 1.6; 2.4 |
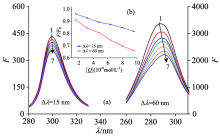
The synchronous fluorescence spectra of LYSO with FLE were shown in Fig.5. It can be seen that the emission maximums of Tyr and Trp residues were slight red shifted in Fig.5(a), which indicated that the hydrophobicity decreased and the polarity around both residues increased. LYSO had six Trp residues and three Tyr residues, which suggested the abundance of higher Trp residues than the Tyr residues in LYSO. In Fig.5(b) (the figure was embedded in Fig.5), it had been shown that the slopes were different between 15 nm and 60 nm, implying that the more opportunities of FLE approaching the Trp residues than Tyr residues.
Fig.7 showed the three-dimensional fluorescence maps of LYSO-FLE system. Peak 1 and Peak 2 are the first and second ordered Rayleigh scattering peaks (λ ex=λ em, λ em=2λ ex), respectively. Peak a was mainly attributed to the spectral behavior of Trp and Tyr residues, when protein was excited at 280 nm, and it primarily disclosed the intrinsic fluorescence of Trp and Tyr residues and the fluorescence from Phe residue can be negligible[10]. Peak a decreased after FLE addition, which indicated that FLE had a combination effect with LYSO. The fluorescence intensity of peak b decreased after FLE addition in FLE-LYSO system, which suggested that the peptide strand structure of LYSO also changed. It can be concluded that the FLE not only could form a complex with LYSO but also change its microenvironment and conformation, which was consistent with the static quenching mechanism.
Circular dichroism (CD) studies were performed in the presence of different FLE concentrations (the figure was embedded in Fig.6). It can be shown that the CD spectra of free LYSO exhibited two negative bands at 208 and 222 nm, which were characteristic of an α -helical structure of proteins[7]. The CD signal increased after the addition of FLE, which indicated that the binding interaction of FLE with LYSO induced changes in the secondary structures of the protein. The secondary structural elements of pure LYSO and its conjugate system were calculated. As shown in Fig.6, the α -helix content of FLE-LYSO system was decreased from 21.1% to 8.8%, and it implied that the addition of FLE would intensified the LYSO skeleton. In short, the binding interaction between FLE and LYSO partially induced the micro-environmental changes of LYSO.
Table 3 revealed the estimated binding energy (Δ G), the inhibition constant, the intermolecular energy, vdw+Hbond+desolvo energy, and the electrostatic energy. It can be seen that the negative Δ G indicated that the formation of complex (FLE-LYSO) was spontaneous. vdw+Hbond+desolvo energy was the main energy, implying that van der Waals and hydrogen bonding forces were the main forces. In addition, the electrostatic forces and other forces also existed between FLE and LYSO, which was consistent with the conclusion of thermodynamic analysis.
| Table 3 Information of the lower energy-ranked FLE-LYSO conformation |
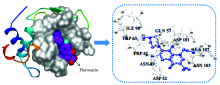
The best energy ranked docking results of FLE-LYSO system were presented in Fig.8 and Table 4. There were nine amino acid residues that took part in the binding interactions of LYSO with FLE. These amino acid residues were ASP 52, GLN 57, ASN 59, TRP 62, TRP 63, ILE 98, ASP 101, ASN 103, ALA 107. In addition, TRP 62 and TRP 63 that were lying at the active site hinge region between α and β -domains were responsible for most of its intrinsic fluorescence[11], and two key active-site residues of lysozyme are GLU 35 and ASP 52[4]. According to the results of docking, the binding FLE occupied the active site, which can competitively inhibit the LYSO activity.
| Table 4 The distance between FLE and atoms of amino acids involved in the binding force |
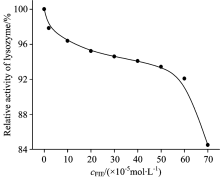
The function of a protein was related to the structure, meaning that the executive function was supported by fixed structure[6]. From the above results, it had been proved that FLE can damage the structure of lysozyme, so we supposed that FLE can not affect the function but also affect the normal physiological function of lysozyme, and lead to metabolic disorders[6]. In Fig.9, the activity of lysozyme decreased with the increasing concentration of FLE, which indicated that the increasing FLE could interact with lysozyme and effect lysozyme activity. These results indicated that structural changes in lysozyme can cause the inhibition of lysozyme activity[6].
In this paper, the interaction of FLE with LYSO was investigated by spectroscopic methods and molecular docking under physiological conditions. Fluorescence quenching between FLE and LYSO was a static quenching process, and the binding process was spontaneous in which van der Waals and hydrogen bond interactions played a major role. The UV-Vis spectra and synchronous fluorescence spectra indicated that FLE was able to interact with LYSO and change the polarity around and the conformation of both residues. Furthermore, CD spectra and three-dimensional fluorescence spectra revealed that the secondary structures of LYSO were altered, which may affect physiological functions of LYSO. Based on molecular docking, FLE was mainly bound to the active site hinge region where ASP 52, TRP-62 and TRP-63 were located, which can competitively inhibit the activity of lysozyme. FIE made the activity of lysozyme decrease with the increasing concentration of FIE. These results offered more detailed information to clarify the molecular toxic mechanism of FLE, potential risk to cause structural damages and the disturbance of normal biological function of LYSO. Such insights were beneficial to unravel the toxicity mechanism in vitro and the risk assessment of drug residue as environmental contaminants.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|