Carbocisteine, also known as carbocysteine, is a mucolytic that reduces the viscosity of sputum and thus can be used to help relieve symptoms of chronic obstructive pulmonary disorder and bronchiectasis by allowing the sufferer to expel sputum more easily. A total of three new metal complexes of carbocysteine, HCcy with the metal ions Sr(Ⅱ), Ba(Ⅱ), and Pb(Ⅱ) have been successfully prepared in alkaline medium in situ H2O/CH3OH (50/50 w/w). The complexes obtained are characterized quantitatively and qualitatively by using micro elemental analysis, FTIR spectroscopy, UV-Vis spectroscopy, X-ray powder diffraction spectroscopy,1H-NMR, and conductivity measurements. From the spectral study, all the synthesized Ccy complexes obtained as monomeric structure and the metals center moieties are six-coordinated except lead(Ⅱ) complex which existed as a four-coordinated respectively, suggesting formulas [Sr(Ccy)(H2O)2], [Ba(Ccy)(H2O)2] and [Pb(Ccy)] in neutral form. Beside, regarding both Sr(Ⅱ) and Ba(Ⅱ) complexes, the aquo groups are existed inside the coordination sphere. The infrared assignments reveal that HCcy ligand act as a bidentate ligand with the metal ions through oxygens of the deprotonated carboxylic COOH group. The1H-NMR spectrum of the [Ba(Ccy)(H2O)2] complex has an absent of the proton of —COOH groups upon the deprotonated of carboxylic group.
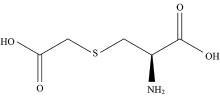
Carbocysteine or carbocisteine having the empirical formula C5H9NO4S, (Fig.1) is one of the most therapeutically prescribed expectorants, sold under the brand name viz., Mucodyne, Rhinathiol and Mucolite[1]. In pediatric respiratory pathology, it can relieve the symptoms of obstructive pulmonary disease and bronchiectasis. On the consideration of its extensive pharmaceutical usage and medicinal value, it has been investigated its chemical structure and composition by employing various spectral techniques like 1H, 13C-NMR, FTIR, Raman, UV-Visible spectroscopy and powder X-ray diffraction method[2]. Density Functional Theoretical (DFT) studies on its electronic structure is also carried out. Drug docking studies were carried out toascertain the nature of molecular interaction with the biological protein system. Furthermore, theoretical Raman spectrum of this molecule has been computed and compared with the experimental Raman spectrum. The forbidden energy gap between its frontier molecular orbitals, viz., HOMO-LUMO is calculated. Atomic orbitals which are mainly contributes to the frontier molecular orbitals were identified. Molecular electrostatic potential diagram has been mapped to explain its chemical activity. Based on the results, a suitable mechanism of its protein binding mode and drug action has been discussed.
A new silver-carbocysteine (Ccy-Ag) complex [Ag2(Ccy)2(H2O)2] has been synthesized and characterized by using a combination of FTIR, Raman, molar conductivity, 1HNMR, electronic spectra, thermal analyses, X-ray powder diffraction (XRD) and scanning electron microscopy (SEM)[3, 4]. The infrared spectrum of Ccy-Ag complex in comparison with carbocysteine ligand prove that Ccy behaves as monobasic bidentate chelate to the silver metal ions via the deprotonated carboxylate O atom. The assessments of Ccy and its complexation with Ag+ in treating Chronic obstructive pulmonary disease (COPD), evaluating immune activities through measuring IL-8, TGFb1, VEGF and TNF-a, antioxidant activities of (Ccy-Ag) complex by measuring SOD, MDA and GPX and bronchial asthma were discussed.
Carboxylates are a very important class of ligand in bioinorganic chemistry. This can be accredited to the versatility of the RCOO-ligand and the wide range of coordination modes that it can adopt. Many carboxylate complexes have been characterized and the coordination chemistry of carboxylic acids is well documented [5]. In metal carboxylate complexes the cationic metal centers (Mn+) combine with the anionic carboxylate groups (RCOO-). The bonding can range from ionic to polar covalent with the physical and chemical properties being dependent on the nature of the R group[5]. The carboxylate functional group has four lone pairs of electrons on the two oxygen atoms which are available for metal binding. These lone pairs can be divided into syn- and anti-lone pairs and are separated by ca. 120° . It has been suggested that the syn-lone pairs are more basic than those in the anti-position[6].
Carbocysteine is an antioxidant and mucolytic agent, is effective in reducing the severity and the rate of exacerbations in COPD patients[7]. The clinical efficacy of carbocysteine seems to be more related to its antioxidant and anti-inflammatory effects than to its mucolytic activity[8]. Metal chelation by carbocysteine represents a very interesting subject not only for environmental chemists, but for biochemists too, because this bio-ligand may modify bioavailability of some metals in vivo. Also, unfortunately, to our knowledge, few papers report for this ligand[9, 10, 11] dealing solution studies. The present investigations involve the synthesis, characterization and structure determination of the solid complexes of carbocysteine with non-transition metal ions such as strontium(Ⅱ), barium(Ⅱ) and lead(Ⅱ). Different tools as elemental analysis, electronic absorptions IR, UV-Vis, X-ray and 1H-NMR spectroscopy have been carried out on some complexes.
All chemicals used (strontium(Ⅱ) chloride hexahydrate, barium(Ⅱ) chloride dihydrate and lead(Ⅱ) acetate trihydrate) were of the purest laboratory grade (Aldrich Chemical Company) and the carbocysteine drug was received from Egyptian International Pharmaceutical Industrial Company (EIPICo.). Doubly distilled water and methanol were employed as solvents. All used reagents were of analytical grade and used without further purifications. Strontium(Ⅱ) chloride hexahydrate, barium(Ⅱ) chloride dihydrate and lead(Ⅱ) acetate trihydrate (1 mmol) were dissolved in 20 mL of water/methanol (50/50 w/w) and then the prepared solutions were slowly added to (1 mmol, 30 mL) of water/methanol (50/50 w/w) with of HCcy ligand solution under magnetic stirring. The pH of solution adjusted to 7~8 by addition of few drops of 10% ammonium hydroxide solution. The resulting solutions heated at 70 ℃ for 2 hrs and left to evaporate slowly at room temperature overnight. The obtained precipitates were filtered-off, wash with hot water and then dried at 80 ℃ and stored under vacuum over anhydrous CaCl2.
The C, H and N percentage determined using Vario EL Fab. CHNS. Metal content and water percentage were determined by gravimetrically technique. IR data for Sr(Ⅱ), Ba(Ⅱ) and Pb(Ⅱ) carbocysteine complexes were measured using infrared Bruker spectrophotometer ranged between 400~4 000 cm-1. The conductance measurements with concentration of 10-3 mol· L-1 for complexity in dimethyl sulfoxide solvent measured using HACH conductivity meter model. 1H-NMR was recorded as dimethyl sulfoxide solutions on a Bruker 600 MHz spectrometer using tetramethyl silane as the internal standard. The electronic absorption spectra were recorded in DMSO solvent within 900~200 nm range using a UV2 Unicam UV/Vis Spectrophotometer fitted with a quartz cell of 1.0 cm path length. The X-ray diffraction patterns were recorded on X 'Pert PRO PAN analytical X-ray powder diffraction, target copper with secondary monochromate.
The elemental analysis results of solid white colour of non-transition Sr(Ⅱ), Ba(Ⅱ) and Pb(Ⅱ) carbocysteine complexes are summarized in Table 1. The melting points of the divalent metal(Ⅱ) Ccy complexes are higher than that of the free HCcy drug ligand, revealing that the chelations are much more stable than ligand. The molar conductance values of the Ccy-M(Ⅱ) complexes found to be 20~30 Ω -1· cm2· mol-1 at 25 ℃, which indicate that all the complexes have a non-electrolytic nature. The low conductivity values agree with the absence of anions inside or outside coordination sphere[3].
| Table 1 Analytical and physical data of the carbocysteine complexes |
The formation of the Sr(Ⅱ), Ba(Ⅱ) and Pb(Ⅱ) carbocysteine complexes was confirmed using electronic UV-Vis absorption spectra. It can see that the electronic spectrum of free carbocysteine drug has a characteristic band observed at 290~310 nm assigned to n→ π * electronic transition. In case of the spectra of the synthesized divalent carbocysteine complexes, the band are blue shifted to lower wave length within 260~280 nm, this result clearly indicate that the Ccy ligand coordinate to central metal ions via the deprotonated carboxylic groups which are in accordance with the results of the FTIR assignments.
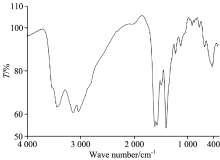
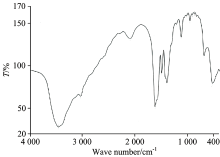
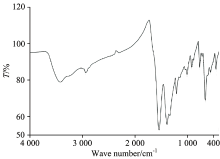
The position of the strong ν (C=O) stretching vibration band in case of the carboxylic acid of the free HCcy drug ligand exhibit at ~1 760 cm-1. Upon complexation to the central metal ions, this ν (C=O) band is absent and the two new bands regarding the asymmetric (νasym(OCO)) and symmetric (νsym(OCO)) stretches appear in the regions of 1 565~1 540 and 1 415~1 390 cm-1, respectively[12]. The magnitude of the separation between these two bands (Δ (OCO) cm-1) has been used, tentatively, as a diagnostic aid in the determination of the nature of the carboxylate coordination[13]. Δ (OCO)=(ν (OCO)asym-ν (OCO)sym) cm-1. Guidelines for diagnosing the type of carboxylate coordination based on the IR spectrum of the metal complex were compiled by Deacon and Philips[14]. The IR spectra of the Ba(Ⅱ)-Ccy, Sr(Ⅱ)-Ccy, and Pb(Ⅱ)-Ccy complexes (Fig.2) show a broad characteristic band at 3 183, 3 034, and 3 050 cm-1 for the stretching vibration motion of — NH2 group[3] and a medium strong bands at (3 442 & 1 610), (3 449 & 1 623), and (3 408 & 1 600) cm-1 for the stretching and bending vibrations of the coordinated water molecules. The absence of large systematic shifts of the ν (NH2) and δ (NH2) bands in the spectra of all the complexes compared with those of the ligand indicates that there is no interaction between the NH2 group and the metal ions. The peaks at 1 565, 1 550, and 1 540 cm-1, for the Ba(Ⅱ), Sr(Ⅱ), Pb(Ⅱ) Ccy complexes respectively, these bands are absent in the spectrum of the free Ccy drug ligand which can be assigned to the asymmetric stretching vibration of the carboxylate group, νas(COO-). While peaks at 1 415, 1 400, and 1 390 cm-1, for the Ba(Ⅱ), Sr(Ⅱ), and Pb(Ⅱ) Ccy complexes respectively, can be assigned to the symmetric stretching vibration of the carboxylate group, νs(COO-). The comparison of ν (OCO)asym and ν (OCO)sym vibrations for the carboxylate function, giving a Δ (OCO) value of 150 cm-1 (Table 2). This value indicates a bidentate coordination mode (Fig.3) for the carboxylate ligand[3, 14], in excellent agreement with those reported for Cu(Ⅱ)-acetate complex.
| Table 2 Infrared spectral bands and assignments of Ccy complexes |
The 1H-NMR spectrum of Ba(Ⅱ) Ccy complex was carried out in DMSO-d6 as a suitable solvent. Upon comparison with the Ccy free ligand, the signal observed at 11 ppm can assigned to the proton of carboxylate group. This signal disappears in the spectrum of the Ccy-Ba(Ⅱ) complex, which confirms the coordination of carbocysteine ligand to the barium(Ⅱ) ion through the deprotonated carboxylic group. On the other hand, the signals of NH2 group which observed at 6.9~7.25 ppm did not affected, which mean that it does not contributed in coordination mode. The band present at 2.50 ppm assigned to the coordinated water molecules, which participate in coordination mode. The results clearly indicate that the ligand coordinate to metal ions via carboxylic (Fig.3) which is in accordance with the results of the FTIR and 1HNMR spectra.
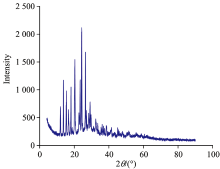
X-ray analysis of Ccy complexes X-ray analysis for Pb(Ⅱ)-Ccy complex was carried out by using X 'Pert PRO PANanalytical diffractometer type, tube anode cupper, and PC-APD, diffraction software indicates that Pb(Ⅱ)-Ccy complex formed crystalline structure (Fig.4), but Ba(Ⅱ)-Ccy and Sr(Ⅱ)-Ccy complexes formed amorphous structure.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|