土壤中的重金属污染会影响农产品品质, 进而对人体的健康产生危害。 土壤中多种重金属元素通常使用化学法进行检测, 需要在实验室使用强氧化性物质对土壤样品进行消解处理, 然后对消解液进行测试。 而X射线荧光光谱法可以实现土壤中多种重金属元素的快速检测, 相对化学法检测, X射线荧光光谱法检出限较高。 对于汞元素来说, 其在土壤中的限值相对其他元素较低, 直接使用X射线荧光光谱法对于低含量的样品难以实现快速检测。 通过设计一个富集装置对土壤中汞进行富集, 并使用X射线荧光光谱仪进行测试, 实现土壤中汞的快速检测, 可以满足实际测试需求。 该装置首先对已准确称量的土壤样品进行加热, 其中的汞元素会发生解吸, 并使用滤膜对解吸出来的汞进行吸附, 从而实现汞元素的富集。 汞发生器会产生特定含量的含汞空气, 使用不同的滤膜进行吸附, 研究发现碳纤维滤膜具有很好的吸附作用, 可以对空气中的汞进行有效富集。 称取相同质量的土壤样品, 在解吸温度为800 ℃的条件下, 使用不同的抽气速率, 并叠加两层膜进行吸附测试。 研究发现随着通过滤膜气流速率的增加, 第一层滤膜的谱峰强度随之降低, 第二层滤膜的谱峰强度随之增加, 结果表明较低的气流流速更有利于滤膜的吸附, 并且使用1 L·min-1的气流流速时, 解吸出来的汞基本全部富集到第一层滤膜上。 通过滴加不同量的含汞溶液到高纯二氧化硅中, 并通过此体系进行富集和测试, 绘制汞的工作曲线, 线性相关系数为0.998 5。 通过对高纯二氧化硅进行多次测量, 可计算检出限和定量限分别为7.52和25.06 ng, 如果此时称样量为0.3 g, 计算可得土壤样品的定量限为0.083 mg·kg-1。 对国家标准土壤样品进行测试, 除低于定量限的一个样品之外, 其余样品的相对偏差不大于11.1%, 表明该方法可以实现农业用地土壤中重金属汞的快速检测。
Biography:NI Zi-yue, (1988—), PhD candidate, Central Iron and Steel Research Institute e-mail: 807407542@qq.com
The pollution of heavy metals in the soil will affect the quality of agricultural products, which could further influence human health. Multiple heavy metals are usually detected with chemical methods in soil, during the process, strong oxidizing materials would be used to digest the samples in the laboratory, and finally, the dissolved solutions are tested. X-ray fluorescence spectrometry could realize the rapid detection of multiple heavy metals in soil, but compared with chemical methods, which has a higher detection limit. For mercury, the national pollution limit is lower than other metals, which makes it difficult to detect rapidly with X-ray fluorescence spectrometry in low-content samples. In this paper, an enrichment device was designed to realize the enrichment of mercury in soil, after testing with the X-ray spectrometer, the rapid detection of mercury in soil could be realized, which met the requirement of the actual test. The soil samples that had been weighed accurately would be heated first, and in this process, the mercury would be desorbed, and at the same time, the filter membrane was used to adsorb it, so as to realize the enrichment of mercury. A mercury generator was used to provide the air with a certain amount of mercury, and different kinds of membranes would be used to study the effects of adsorption. The result found that carbon fiber filter membranes have a good effect on adsorption and could enrich mercury in the air. When different flow velocity was adopted with the same weight of the samples, and the desorption temperature was set up at 800 ℃, the adsorption behavior of two membranes was studied. The results showed that, with the increase of the flow velocity, the intensity of the first membrane decreased, but the intensity of the second membrane increased, which meant that lower flow velocity was a benefit for the adsorption of membranes. when the different amount of mercury contained in solution was added in high-purity silicon dioxide, after enriching and testing by the designed device, the working curve could be obtained with the linear correlation coefficient to be 0.998 5. And the detection limit and quantification limit could be calculated as 7.52 and 25.06 ng respectively when multiple high-purity silicon samples were tested. It meant that if the weight of the sample was 0.3 g, the quantification limit would be 0.083 mg·kg-1 in the soil. The relative deviations were no more than 11.1% for the national standard samples except one sample that was below the quantitative limit, which indicates that this method could realize the rapid detection of mercury in the soil for agricultural land.
Mercury (Hg) is a global environmental contaminant and its major anthropogenic sources emission including nonferrous metal smelting, the burning of fossil fuels and chemical industry.
Atmosphere mercury will accumulate in the soil by wet and dry deposition[1, 2]. Both plant and organisms will be influenced by mercury after which is above certain content in contaminated soil[3]. And mercury could be methylated by microorganisms which will be taken up by organisms, which will accumulate and magnify in the food chain, and eventually leading to detrimental effects on the ecosystem and human health[1].
The quantification of mercury in the soil is commonly realized by using atomic florescence spectrometry (AAS), atomic fluorescence spectrometry (AFS) or inductively coupled plasma mass spectrometry (ICP-MS)[4, 5, 6, 7]. Before tested by those methods, the soil samples should be dissolved into solution with strong acid. Recently, the direct detection of mercury in soils has been realized by atomic absorption spectrometry and atomic fluorescence spectrometry[8, 9]. However, the analyzers could not realize the detection of multiple elements.
The determination of multiple heavy metals in soil has been realized based on energy dispersive X-ray fluorescence (EDXRF), which has been used to access the metal pollution in soils[10, 11, 12, 13, 14]. But there are still some problems in the detection of mercury in soil. According to the risk control standard for soil contamination of agricultural land for soil environmental quality in china, the risk screening values of mercury were 0.5, 0.5, 0.6, 1.0 mg· kg-1 respectively for different pH in paddy soils, and were 1.3, 1.8, 2.4, 3.4 mg· kg-1 in other soils[15]. And the limit detection of mercury is higher than risk screening values when tested by EDXRF. Fiamegos et al. validated the detection of multiple heavy metals in organic and inorganic samples based on EDXRF from sub mg· kg-1 to percentages, and the quantification limit of mercury was 1.7 mg· kg-1 [16]. Brent et al. demonstrated that a handheld XRF device could be used for mercury screening in soils with the detection limit of 7.4 mg· kg-1 [17].
When detected by EDXRF, because the K series lines needed too high energy to irritate, L series lines of mercury was usually chosen as an analytical line. But the intensity of L is lower than the K series lines. And Lα and Lβ 1 have a higher intensity than other lines. However, the L series lines of mercury often interfered with other heavy metals contained in the soils. For instance, Lβ 1 of mercury would be overlapped with the lines of As and Br, which are usually present in the soil. And when W and Ge existed in the soil for the polluted areas, Lα would be interfered with. In the meantime, the matrix effect is strong in the soil. So the limit of detection for mercury is high, which could not meet the requirements of actual test in soils.
In our study, the soil was heated at 800 ℃ for thermal desorption of mercury firstly, and then carbon fiber membrane was used to absorb the mercury. After the process, the membrane which has accumulated mercury from soil was tested by EDXRF, and finally, the content of mercury in soil could be calculated through employing absolute content absorbed in the membrane and the mass of the soil. Because the membranes contained no interfering elements and enriched the mercury in it, the detection limit was lower than detected directly in soil with EDXRF. By this way, EDXRF could be used to estimate whether the content of mercury exceeds standard values in agricultural land.
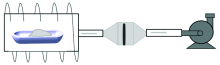
An X-ray fluorescence spectrometer (NCS TESTING TECHNOLOGY CO., LTD, NX-100S heavy metals analyzer) was employed, which was equipped with a 65 W X-ray tube 40 kV/1 000 μ A excitation source, an Ag-anode target and an SDD detector with an energy resolution of 125.3 eV at 5.895 keV (Mn: Kα ). A device with thermal desorption and membrane enrichment system was designed for the further detection of mercury, which could realize the desorption and the enrichment of mercury from soil to the membrane. The sketch picture of the device was shown in Fig.1.
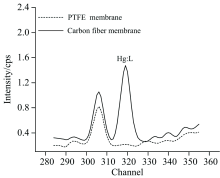
A vapor generator of mercury was used to provide the air with a certain content of mercury in it. The membrane, made of polytetrafluoroethylene(PTFE) and carbon fiber, was used to absorb mercury contained in gases. When the concentration of mercury was set at 12.03 μ g· m-3, the gases were controlled by mass flow controller to get through membranes for three minutes, respectively. After that, the membranes were detected with energy dispersive X-ray fluorescence. The spectral line of mercury was shown in Fig.2. And only carbon fiber membrane has an obvious peak of mercury, which means that it is appropriate for the enrichment of mercury in the air.
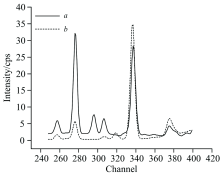
After the temperature of the sample chamber had been heated at 800 ℃ by the furnace, the sample boat filling with soil was put in it. The released mercury accompanying with air was extracted by an air pump. Between the thermal desorption furnace and the pump, carbon fiber membrane was put to absorb mercury. The national standard sample GBW07404 with the mercury content of 0.59 mg· kg-1 was tested both directly with common EDXRF and by the enrichment device. The spectral line of mercury was shown at the channel of 320 in Fig.3. We can see that, when tested directly by common EDXRF, the spectrum of GBW07404 did not have an obvious peak of mercury. After the process of thermal desorption and enrichment, the peak of mercury can be detected in membrane obviously. The results indicate that after enrichment in the carbon fiber membrane, trace mercury in the soil could be detected with common EDXRF.
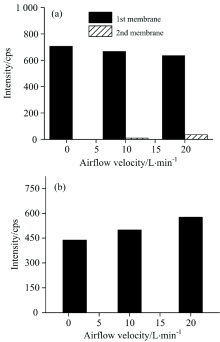
The airflow velocity has an influence on the absorption in carbon fiber membrane. The high-purity silica mixed with the mass of 2 000 ng of mercury in it, put in the sample boat in the thermal desorption system, was used to obtain mixture air containing mercury in it. When the airflow velocity was adjusted to 1, 10 and 20 L· min-1 respectively, after enriching in this system and absorbed by two pieces of membranes with the same sample, three groups of carbon fiber membranes could be obtained. And the membranes were irradiated by EDXRF on the side of the surface (obverse side) which absorbed mercury firstly. As shown in Fig.4(a), the first membrane had a stronger intensity of mercury than the second membrane, which meant that the first filter contained more mercury than the second one and the carbon fiber membrane were of high efficiency to absorb mercury passed through it. The intensity of mercury in the first membrane decreased with the increase of airflow velocity but increased in the second membrane. The result implied that the thickness of mercury absorbed in the filter membrane increased with the increase of airflow velocity. The reverse side of the filter membrane was also detected by EDXRF, and the intensity of mercury increased with the increase of airflow velocity, as shown in Fig.3(b). The result confirmed the speculation and suggested that low airflow velocity should be adopted to obtain a stronger signal for the detection based on EDXRF. Hence, airflow velocity of 1 L· min-1 was chosen for further studying, and all the mercury almost absorbed in the first membrane.
The solutions that are of different concentration of mercury were added in high-purity silica to obtain different samples. Then carbon fiber membrane was obtained, which contained different weight of mercury for the testing by EDXRF. And through adopting different intensity of mercury about different weight, the calibration curve of mercury could be drawn. The linear correlation coefficient was 0.995 8, as shown in Fig.5, which meant that EDXRF could be used to detect the different weight of mercury in samples after thermal desorption and enrichment in the membrane with this device.
11 samples of high-purity silica were tested in this system to obtain the limit of detection for the testing of mercury with EDXRF. And the standard deviation was calculated about the samples. And three times and ten times of standard deviation was taken as detection limit and quantitation limit respectively. The detection limit and quantitation limit was 7.52 and 25.06 ng. If the samples were weighed at 0.3 g, and the content of mercury could be detected at 0.083 mg· kg-1, which meant that this method could meet the requirement of soil environmental quality according to risk control standard for soil contamination of agricultural land in China.
National standard samples were used to test and verify the process for detection of mercury in soils. Samples were weighed first and then put in the thermal desorption and enrichment system to obtain filter membrane which containing mercury in soil. After the membranes tested by EDXRF, the absolute content of mercury could be obtained, and then the concentration of mercury in soil could be calculated. The results were shown in table1, the absolute deviation were no more than 0.083 mg· kg-1, and relative deviations were no more than 11.1% except a low content sample of GSD-1a. It is because when the sample was weighed at about 0.3 g, the total content of mercury was about 9.6 ng, which couldn’ t exceed the quantitation limit of the method.
| Table 1 The accuracy for detection of mercury |
The detection of mercury in soil was realized by adopting the enrichment device with energy dispersive X-Ray spectrometry. When mercury mixed with air was extracted to pass through membranes by an air pump, carbon fiber membrane was found effectively to absorb mercury in air, and the optimized air velocity was chosen as 1 L· min-1 to achieve bigger fluorescence signals. And after mercury transferred from soil to membranes, the detection limit of which could be lowered.
The calibration curve was drawn with high-purity silica mixed solution with different content of mercury in it. The linear correlation coefficient was 0.995 8, the detection limit and quantitation limit was 7.52 and 25.06 ng respectively, which meant that this method could meet the requirement of mercury testing in soil environmental quality for risk control standard for soil contamination of agricultural land in China. National standard samples were used to verify the detection of mercury with EDXRF after thermal desorption and enrichment. The absolute deviations were no more than 0.083 mg· kg-1, and relative deviation were no more than 11.1% except a low content sample of GSD-1a, which meant that this method could realize the rapid detection of mercury in soil.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|