Three types of metal ions barium(Ⅱ), nickel(Ⅱ) and cerium(Ⅲ) complexity of ATN drug have been prepared and characterized using molar conductance method, FT-IR, electronic, and1H-NMR analysis measurements. The chemical and physical results for all atenolol complexes are agreement with the speculated structures. For the divalent (Ba & Ni) and trivalent (Ce) metal atenolol a molar ratio 1:2 was established. Qualitative chemical analysis showed that for the divalent metal complexes, the chloride ions are not involved in the complexes, suggesting that all of these complexes, [Ba(ATN)2]·2H2O and [Ni(ATN)2(H2O)2]·4H2O are neutral. However, for the cerium(Ⅲ) complex, [Ce(ATN)2(NO3)]·3H2O, the nitrate group is existed inside the coordination sphere. ATN make astable metal complexity with barium(Ⅱ), nickel(Ⅱ) and cerium(Ⅲ) ions. Electronic absorption analysis of Atenolol give two fundamental peaks at 225 nm and 274 nm refers to variation in transition electrons of ligand, UV spectral analysis of the three complexity obtained give asymmetric broad band in the range 200~400 nm, the reults are convenient with the suggestion of metal-nitrogen and metal -oxygen bonds. The infrared analysis data proved that ATN act as bidentate ligand through the N atom of the —NH group and O atom of the deprotonated alcoholic OH group. Nickel(Ⅱ) and cerium(Ⅲ) complexity make six-coordinate geometry, whereas the barium(Ⅱ) complex exhibit four-coordinate geometry. Ni(Ⅱ)-ATN complex has an effective magnetic moment equal 3.12 B.M, that is assigned to octahedral structure. The1H-NMR spectral results of Ba(Ⅱ)-ATN complexity give strong signal at ~4.00 ppm due to protons of —CH2 that influenced by low degree due to complexity. These results confirm the position of chelation through the N atom of the —NH group and O atom of the deprotonated alcoholic OH group.
New complexity of vonadyl(Ⅱ ), zinc(Ⅱ ), paladium(Ⅱ ), gold(Ⅲ ) and platinum(Ⅳ ) with atenolol anti-hypertensive drug were prepared and explained using C, H, N analysis, magnetic measurments, UV-Vis, infrared, electron spin resonance, 1H-NMR spectroscopy, X-ray diffraction, scanning electron microscopy (SEM), and TG-DTG. The infrared data proposed that atenolol act as bidentate ligand towards the M+n with N of 2nd NH2 and O of hydroxyl group after loss of proton. According to elemental analysis data, the molar ratio is 1:2 (M:ATN). From literature survey, it is concluded that when bioorganic ligands introduced as transition metal complexity, its activity increased and highe therapeutic activity were produced[2, 3]. The disclosure and advancement of unused metallo-drugs stay an ever developing zone of investigate in application of therapeutic bio-inorganic chemistry[4, 5, 6], that can be separated into two fundamental types: 1st, drug ligands which react with metal particles; and 2nd, metal-based drugs and imaging specialists where the metal act as central particle is more often than not the key include mode of action[7]. The treatment applications of metal complexity counting for cases: bleomycin anti-microbial causes DNA strand breakage 4), through formation of an intermediate metal requiring a metal particle cofactor such as Cu for making more action within cancer treatment[8]. Anti-microbial drugs of the tetracycline type are coordinators of Ca2+ and Hg2+ particles. Rh poly amino carboxylate complexity (Ru-pac) have cysteine protease hindrance action, these complexity act as metallo-inhibitor specialists for diseases[9]. The cisplatin, carboplatin were the 1st and 2nd platinum drugs generation individually, which were broadly utilized within the treatment of cancer[10]. On the base of the relationship between platinum(Ⅱ ) and paladium(Ⅱ ) complexity, a few considers of palladium compounds as appropriate drugs have been carried out[11]. Geometry and complexity shaping forms of palladium (Ⅱ ) are exceptionally comparable to those of platinum(Ⅱ ) hence it was conjectured that Pd complexity may too have anti-tumor effects and serve as great models for the understanding of more inactive p(Ⅱ ) anticancer drugs[12, 13, 14, 15]. The gold(Ⅰ ) complexity act as anti-arthritic drugs, while they too hinder the development of tumor cells “ in-vitro” and numerous have anti-mitochondrial effects[16, 17, 18].
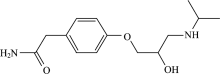
The physiological effects of anti-hypertension of a few amino alcohols that behavet as β -blockers was cause for their generally large utilize practically within the final a long time. However, due to the nearness of NH2 and hydroxyl groups as they are dynamic givers particularly reasonable for chelation of bio vital metals like Cu and Zn[19, 20, 21, 22, 23, 24]. Atenolol (ATN; Fig.1), chemical formula is C14H22N2O3[25], could bea β 1-(specific for cardio) adreno-receptor adversary medicate broadly utilized within the treatment of hypertension. It is additionally utilized in administration of headache prophylaxis and hyperthyroidism[26, 27]. The distinctive strategy procedures adjusted to the examination of atenolol have been detailed[28, 29, 30, 31, 32, 33, 34, 35]. But till now, There is no attempt in the literature have been made.
Atenolol used in this paper was received from the Aldrich Chemical Company. All of the other chemicals (BaCl· 2HO, NiCl· 6HO and Ce(NO3)3· 6H2O were of high degree of purity.
For ATN complexity, the Ba(Ⅱ ), Ni(Ⅱ ), Ce(Ⅲ ) complexity were prepared by reaction of BaCl· 2HO, NiCl· 6HO or Ce(NO3)3· 6H2O metal ions with (1 mmole) in 20 mL distilled water to dissolved ATN (20 mL of absolute CH3OH) with stoichiometric 1:2. The pH of atenolol complexity were in between ~8.0~9.0 using 5% NH4OH. The resulting solutions were stirred and refluxed at 60 ℃ for 2 hr. The precipitates produced, filtered off and washed several times with (distilled water absolute CH3OH). The precipitates were dried at 80 ℃ under vacuum over anhydrous calcium chloride.
C, H and N analyses in Vario EL Fab. CHNS. The amount of water and metal calculated gravimetrically. IR spectra of the complexity were measured using Bruker IR spectrophotometer ranged from 400~4 000 cm-1. All the analysis were carried out at room temperature. UV spectrum of the complexity were carried out in dimethyl sulfoxid solvent with 1× 10-3 mol· L-1 concentration, ranged from 200~800 nm using Unicam UV/Vis spectrometer. The mass susceptibility (Xg) of nickel(Ⅱ ) complex was measured at room temperature using Gouy’ s method by a magnetic susceptibility balance from Johnson Metthey and Sherwood model. 1H-NMR was recorded as DMSO solutions on a Bruker 600 MHz spectrometer using TMS as the internal standard.
The analytical physical results of the ATN complexity were in Table 1. C, H and N show that the Ba(Ⅱ ), Ni(Ⅱ ) and Ce(Ⅲ ) complexity with ATN were of 1:2 (M+n:atenolol) stoichiometric ratio. Ba(Ⅱ ), Ni(Ⅱ ) and Ce(Ⅲ ) synthesized complexity have different colors. ATN complexity are partially soluble in hot CH3OH, DMSO and DMF. The proposed structural formula of the prepared complexity were based on the data of: (C, H, N), molar conductance, (IR, electronic) spectra, magnetic measurements, as well as the thermo gravimetric analysis (TG - DTG).
| Table 1 Analytical and physical data of Ba(Ⅱ ), Ni(Ⅱ ), and Ce(Ⅲ )-ATN complexes |
Electronic absorption analysis of Atenolol give two fundamental peaks at 225 and 274 nm refers to variation in transition electrons of ligand[41]. Electronic absorption data for complexity were occurred in dimethyl sulfoxide to clarify the solvent effect. UV spectral analysis of the three complexity obtained give asymmetric broad band in the range 200~400 nm (the bands obtaines are weak and low energy). The reults are convenient with the suggestion of metal-nitrogen and metal-oxygen bonds[41]. UV analysis of all complexity has primarily ligand centered transition bands with large absorption in the range 300~250 nm. The benzene rings bands appeared at higher energies and attributed to π—π* transitions[43]. The solid reflectance spectrum of the nickel(Ⅱ ) complex showed bands at 650 and 460 nm that assigned to 3A2g(F)→ 3T1g(F)(ν 2) and 3A2g(F)→ 3T1g(P)(ν 3) transitions, respectively, therefore an octahedral geometry predicted[42].
Atenolol has high intense two intermolecular H- bonds comes from (amino and carbonyl groups) and (hydroxyl and 2ndry amine) groups[43]. The IR spectrum of atenolol (Fig.2, Table 2) has two peaks at 3 356 and 3 174 cm-1 referred to stretching frequencies of (OH, NH2 and amide NH groups), no single peak refers to (OH) appeared in the spectrum of ligand. The IR peak at 1 639 cm-1 with the shoulder at 1 670 cm-1 is attributed to stretching frequencies of carbonyl group[44, 45]. The stretching vibration of (NH) disappeared in the infrared or Raman spectrum, which can be attributed to the intermolecular hydrogen bonding effects which are expected to decrease the vibration of this band and appeared in the broad peak at 1 639 cm-1 in the IR spectrum. This suggestion is proved by compare the infrared data of ATN derivatives like metoprolol, another β -blocker which have same geometry of ATN but no NHCO groups, at 1 634 cm-1 that can be attributed to ν (NH) mode since this compound not has the C=O group[46]. By compare the IR spectra of Previous studies[47, 48, 49, 50, 51, 52] of the free atenolol, the IR spectra of atenolol complexity [Fig.2(b, c)] contain the most important vibrations of atenolol. The main infrared spectral band data are in (Table 2) and according to the following facts.
| Table 2 IR absorption frequencies (cm-1) of ATN complexes |
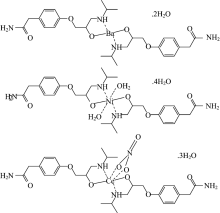
ATN has peak at 3 174 cm-1 refers to 2nd stretching vibration (NH) is shifted in the spectra of the complexity, which confirms the participation of N atom of the amino alcohol of ATN in the complexity with M+n ions. An important bands of the ATN refers to stretching frequencies of carbonyl and amino groups, refers to it is strong and sensitive to ATN geometry. Due to partial overlapping of the peaks attributed to these stretching frequency, will make the doublet structure of spectra[53]. This band shifted to lower frequency in the complexity, confirming that NH group getting into the chelation process. The spectrum of (carbon-oxygen) of OH group of atenolol at 1 037 cm-1 decreased in frequency by complexity, confirming that alcoholic group getting into in the chelation process[54]. The as (NH2) group appeared in free atenolol at 3 356 cm-1. Asymmetric stretching vibration of (NHCO) by complexity is split and appeared at higher frequency in comparison with comparative bands in free atenolol at 3 363~3 324 cm-1 due to H-bond[55]. In the atenolol ligand spectrum the band due to to (OCNH2) vibration is appeared at 1 298 cm-1, after complexation, this band remains unshifted, which indicated that N of the NHCO group not participate in coordination process. The stretching vibration of (C—C ring) at 1 612 cm-1 remains constant in free ligand. The same for (CH2) and ring breathing bands at 1 244 and 886 cm-1, respectively. The low intensity peaks at 600~400 cm-1 are due to stretching vibration of (metal-nitrogen and metal-oxygen)[55]. The above results proved a bidentate of chelation of atenolol with M+n ions through deprotonated hydroxyl and 2ndry NH group from the amino alcohol moieties of two moles of atenolol ligand (Fig.3).
Magnetic measurements were carried out according to the Gauy Method. The square planar geometry concerning Ni(Ⅱ ) complexity is a diamagnetic, while, tetrahedral complexity have magnetic moments in the range of 3.20~4.10 B.M. but when the nickel(Ⅱ ) complexity have an octahedral geometry the magnetic moment located in the range of 2.90~3.30 B.M.[56, 57]. Therefore Ni(Ⅱ )-ATN complex has an effective magnetic moment equal 3.12 B.M, that is assigned to octahedral structure.
The 1H-NMR spectral results of the atenolol ligand in (DMSO-d6) has a sharp signal t at 4.916 ppm due to the hydroxyl OH proton, this peak didn’ t appear in the spectra of the Ba-ATN complexity, which confirm the loss of proton of the hydroxyl group. The proton of the —OCHn was deshielded in Ba(Ⅱ ) complexity due to high electronegativity of the oxygen of hydroxyl group. The Ba(Ⅱ )-ATN complexity give a sharp singlet signal at 1.047~1.156 ppm refers to six protons of two CH3 group confirming that these protons are magnetically equivalent. In the complexity the protons of —CH, produced as a triplet signal at 2.71~3.27 ppm and a strong signal at ~4.00 ppm due to protons of —CH2 that influenced by low degree due to complexity. These results confirm the position of chelation which discussed above.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|