Hydroquinone (HQ) is an phenolic aromatic compound used in cosmetic medicine as skin lightening material since long time ago. Accordingly the several pathological conditions that observed, the use of HQ for this purpose was recently contraindicated. The commonly noted health problem associated with hydroquinone use was contact dermatitis. The present study was designed and conducted to determine the HQ presence and concentrations in ten samples of skin lightening cosmetics available in the local market. GC-MS analysis was employed for detection of HQ qualitatively while HPLC analysis was used as a quantitative analysis for determination of the HQ concentrations in each sample. The results obtained indicated that the overall range of HQ levels in all respected samples ranged from 0.003 9% to 0.14%.
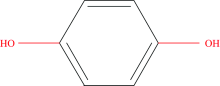
Hydroquinone is one of the aromatic organic compounds of the phenolic family whose chemical formula is C6H4(OH)2 (Fig.1). The chemical composition of HQ includes two groups of hydroxyl linked to a benzene ring at a para position and has very weak acidic hydroxyl groups. It can be losses H+ from one of the two hydroxyl groups to create a mono phenolate ion or H+ loss from both hydroxyl groups together to form bis phenols. The appearance of hydrocarbons in E. coli is attributed to the demolition of tyrosine and other similar aromatic substances. HQ as a diffuse molecule and is an oxidation product of some aromatic compounds[1]. It is naturally found in plants, foods and coffee[2, 3], it has been applied as a skin whitening agent. Several scientific experiments have proven the effectiveness of (HQ), which makes it a remedy[4]. HQ is used as an antioxidant in the photographic industry as well as a dye removal agent in cosmetics such as skin softening creams[5], but it affects human health, such as leukemia (leukoderma-encofette), external synchronization, and long-term can become carcinogenic[6, 7]. HQ is a highly toxic substance used in cosmetics, as it is a commonly used ingredient in facial cleansers and moisturizers[8]. The action mechanism of HQ in the biological process depends on the inhibition of the formation of melanin. The European Union regulation specified its content in cosmetics within the 2% level, and analyzed creams for skin color show its presence and some of its derivatives[9, 10, 11]. The conceivable explanation for this work is due to its inhibitory effect on tyrosinase, which has not yet been confirmed[12]. However, due to frequent side effects, medical surveillance is necessary, so it explains why products containing HQ used for cosmetic purposes are banned in the USA, the European Union and many African and Asian countries[11, 12]. As a result, it is recommended not to use it in cosmetics[13, 14]. The use of hydroquinone in cosmetics was not authorized in the European Union in 2001, and products used to treat abnormal and those containing this compound were not classified as cosmetics but as clinical preparatory drugs containing 2%~4% HQ prescribed for the treatment of hyperparathyroidism and pigmentation such as freckles and Melissa[15]. HQ is widely known for its toxic nature in ecosystems arising from its frequent use in applications such as pesticides, medicines, cosmetics and dyes[16, 17]. Accordingly, various analytical methods were used to detect HQ, which include chromatography, chemiluminescnce spectrophotometer and electrochemical methods[18]. This study was designed and conducted to determine the HQ presence and concentrations in ten samples of skin lightening cosmetics available in the local market using GC-MS and HPLC analyses.
Ten skin lightening creams were collected randomly form different cosmetic markets in Khartoum (Sudan).
A Shimadzou GC-MS (TQ8040) was used with capillary column (30 cm×0.25 mm×0.25 μm), (5% phenyl-95% dimethyl), carrier gas helium, constant flow 1 mL·min-1, temperature program 0.7 min at 90:35° /min to 240:8° /min 290:25° /min to 325° -6 min final hold[20]. A Shimadzu LC-9 technique was employed with UV detector set at 295 nm having ODS column (25 cm×4.6 nm). Samples were adjusted at (35±1) ℃. The mobile phase used was a mixture of water:ethanol (45:55) with the flow rate of 1.5 mL·min-1 [19].
Three grams of each cosmetic cream was weighed and placed in a 100 mL beaker. 12 mL ethanol (HPLC grade) was added to each sample. The contents of each sample was thoroughly mixed and homogenized in the water bath at 65 ℃ for 15 mins for complete separation of fats. The mixture was then cooled in an ice bath and filtered. The filtrate was subjected to GC-MS analysis[19]. To prepare the reference solution, an amount of 0.1 gram of HQ was dissolved in 0.04 mL of ethanol in the 10 mL volumetric flask. The final volume was completed to the 10 mL. For each analysis process, the standard solution was freshly prepared due to its instability at room temperature for more than one day.
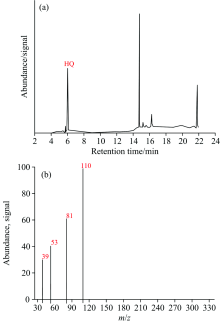
For each sample, 1 μL was injected and chromatography run was made. The chromatogram data was recorded. The retention time (Rt) was measured for each sample and compared with that of the standard solution. The Rt values were calculated for each sample and their results are given in Figure 2 and Table 1.
| Table 1 Rt & M/z values of analyzed samples |
Two grams of each sample was accurately weighed and placed into a beaker. An amount of eight ml of the mobile phase (water:methanol mixture 45:55) was added to the sample. The contents in the beaker were thoroughly mixed and homogenized in a water bath at 65 ℃ for 15 mins. The mixture was then cooled in an ice bath to separate the fats and waxes. Filtration of these mixtures was employed and the filtrate transferred to 10 mL volumetric flask. The volume was completed to 10 mL using the mobile phase. An amount of 0.01 gram of HQ was weighed and put into 10 ml volumetric flask. The content of the flask was vigorously dissolved in small amount of the previously prepared mobile phase. The volume was completed to 10 mL. One ml of this solution was transferred into 10 mL volumetric flask and again diluted up to the mark of the flask using the mobile phase.
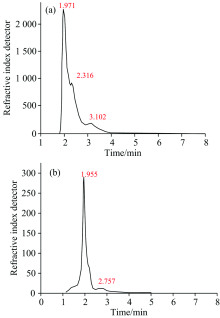
For each sample, 20 μL of sample solution was injected for chromatography. The peak area for each sample was measured. A comparison between standard solution and sample solutions peaks was made. The total amount of HQ was calculated as percentages in Figure 3 and Table 2.
| Table 2 Percentage concentration of hydroquinone compound |
Ten samples of skin lightening creams were randomly selected from Sudanese markets to determine the HQ presence and concentrations. The samples were analyzed by using gas chromatography mass spectrometry and high performance liquid chromatography. All samples which were checked by chromatographic methods show positive results for hydroquinone presence, and noticed that there is no sufficient warning on the products labels explain the risk of using this creams especially it’ s available for sale in the markets. Also the results obtained indicated that the overall range of HQ levels in all samples ranged from 0.003 9% to 0.14%.
This research was funded by the deanship of scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding program.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|