傅里叶变换微波光谱仪是测量分子转动跃迁的主要工具, 是研究分子转动光谱学的重要仪器。 以量子力学为基础的转动光谱学对物质分子的结构分析以及破解射电望远镜所捕获的深空分子信号至关重要, 这使得微波光谱仪在相关领域能发挥不可或缺的作用。 目前, 世界各国都在致力于研制和改进微波光谱仪以提高仪器的分辨率、 灵敏度、 以及应用范围, 我国也正在该类仪器研制上进行积极的探索, 期望为该领域做出应有的贡献。 介绍了一种工作频段在1~18 GHz的宽带啁啾脉冲式傅里叶变换微波光谱仪的设计和研制。 该光谱仪用于线性频率扫描的宽带啁啾脉冲信号由采样速率为1.25 GS·s-1的任意波形发生器产生。 宽带啁啾脉冲信号经混频和放大后可覆盖特定的频率范围, 随即通过喇叭天线传播到样品真空室与超音速膨胀的气相样品分子束相互作用。 样品分子被激发后发出的分子自由感应衰减信号由接收电路导出并放大, 然后直接在高速数字示波器上数字化。 该微波光谱仪的诸多电子器件均由计算机控制, 利用开发的LabVIEW程序可实现仪器的自动化控制。 应用气体喷嘴技术能有效降低待测样品气体束在检测室的转动温度, 使仪器获得更好的检测灵敏度。 应用多脉冲自由感应衰减技术能大幅度提高信号采样频率从而进一步提高仪器灵敏度。 利用实验室研制的啁啾脉冲式傅里叶变换微波光谱仪对盐酸和叔丁醇的化学反应进行了监测, 并成功检测到了该反应的产物叔丁基氯。 通过测量天然丰度下反应产物叔丁基氯及其单取代37Cl同位素异数体的分子转动光谱数据, 并利用光谱分析软件拟合这些数据后得到了叔丁基氯精准的光谱参数(转动常数, 离心畸变常数, 核四极耦合常数等)和分子结构信息。 将以上参数和结构信息与高斯计算结果对比后证实了本实验宽带光谱仪检测到的光谱数据具有高精准度。 通过对比前人所测的光谱数据进一步展示了该实验宽带光谱仪在低频范围内杰出的测试性能。
Biography: JIAO Chao, (1995—), doctoral student in Nanjing University of Science and Technology e-mail: cjiao@njust.edu.cn
Fourier transform microwave spectrometer is the main tool for measuring molecular rotational transitions and an important instrument for researching molecular rotational spectroscopy. Based on quantum mechanics, rotational spectroscopy is essential for the structural analysis of molecules and for deciphering molecular signals from deep space captured by radio telescopes, thus making microwave spectrometers indispensable in those fields. At present, researchers from countries all over the world are working on the instrumentation of microwave spectrometers to improve the resolution, sensitivity, and application range as well, while Chinese researchers are also exploring such instrument development actively, and expect to make due contributions to those fields. In this paper, the design and development of a chirped-pulse Fourier transform microwave spectrometer are presented with a frequency coverage of 1~18 GHz. The broadband chirped pulse of a linear frequency sweep is generated by the arbitrary waveform generator with a sampling rate of 1.25 GS·s-1. After mixing and amplification, the chirped pulse with a certain frequency coverageis broadcast through a feedhorn antenna into the vacuum chamber, where it interacts with a supersonically expanded molecular beam. The free induction decay (FID) signal emitted by the excited molecules is induced and amplified by the receiving circuit and then directly digitized on a high-speed digital oscilloscope. Many electronic devices of the microwave spectrometer are controlled by a personal computer, and their automatic operation can be achieved by a LabVIEW program. The gas nozzle technology is applied to improve the sensitivity of the spectrometer by effectively reducing the rotational temperature of gas samples in the vacuum chamber. Multiple free induction decay (multiple FID’s) technology is also applied to further improve the sensitivity by dramatically increasing the signal sampling rate of the spectrometer. By using this broad-band chirped-pulsed Fourier transform microwave spectrometer developed in the laboratory, a chemical reaction of hydrochloric acid and tertiary butanol was monitored, with the reaction product tert-butyl chloride detected successfully. The rotational spectra of tert-butyl chloride and its singly-substituted37Cl isotopologue were measured in their natural abundance, and were then fit by the spectrum analysis software to provide accurate spectral parameters (rotational constants, centrifugal distortion constants, and the nuclear quadrupole coupling constants, etc.) and molecular structure. The high accuracy of spectral data measured by the spectrometer was proved by comparison with Gaussian calculation. The spectrometer’s excellent performance in the low frequency range was also demonstrated when compared with the spectral parameters measured by predecessors.
Rotational spectroscopy is an extremely effective technique for the study of gas-phase molecular structure, which is mainly used to study the basic aspects of molecular physics. The rotational spectra of molecules are highly sensitive to small structural changes. When fine or hyperfine structures are observed, the electronic structure information of molecules can be derived with this technique, such as nuclear quadrupole coupling.In radio astronomy, this technique also plays a key role in exploring the chemical compositions of interstellar media[1].
Microwave spectrometer is the main instrument for researching rotational spectroscopy.Since invented in 2006[2], the broadband chirped-pulse Fourier transform microwave (CP-FTMW) spectrometer has revolutionized the field of microwave spectroscopy.Due to the advance in high-speed digital electronics technology for microwave signal generation and detection, the development of broadband CP-FTMW spectrometer has dramatically expedited the speed of acquiring sensitive and high-dynamic broadband microwave spectra[3]. In a single scan of the spectrometer, the bandwidth of collected spectrum can be over 10 GHz, which is several orders of magnitude faster than before, and the research scope of rotational spectrum is also expanded[4]. There are a variety of entirely new experiments arising from this technique, from using it to measure chemical dynamics and reaction states[5] to dual-resonance experimental multiplexing[6, 7].
In this report, a broadband CP-FTMW spectrometer is introduced, with frequency region ranging from 1~18 GHz, single scanning of 1 GHz, resolution of 5~50 kHz. The gas nozzle technology is used to effectively reduce the rotational temperature of the gas sample, so that the instrument can obtain best observation sensitivity. Multiple free induction decay (multiple FID’ s) technology[8] is applied to further improve the sensitivity of instrument by dramatically increasing therate of signal sampling. To demonstrate the performance of the broadband CP-FTMW spectrometer, the rotational spectra of tert-butyl chloride, the product of a chemical reaction between tert-butanol and HCl, were detected to accurately determine its structure and spectroscopic constants.
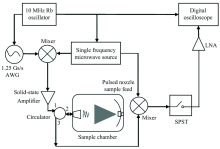
A schematic diagram of the 1~18 GHz CP-FTMW spectrometer is shown in Fig.1.
One single scanning bandwidth of this microwave spectrometer is 1 GHz in the working frequency range of 1~18 GHz. The schematic is separated into three sections: (a): chirped-pulse generation and amplification; (b): sample vacuum chamber; (c): FID detection. (a): A chirped-pulse, typically with bandwidth of 500 MHz and duration of 5 μ s, is created by an arbitrary waveform generator (AWG, TE Tabor Electronics, WX1281C) with sampling rate 1.25 GS· s-1. The chirped-pulse is then mixed with a single-frequency microwave signal generated by the signal generator (ROHDE& SCHWARZ SMF 100A) to up-convert into 1 GHz target frequency band in the working frequency range. After the mixed pulse signal is amplified by a solid-state amplifier (BONN Power Amplifier 1018-5M), and broadcast through a circulator (TDK8916E1B7, 1→ 2) as well as a double-ridged horn antenna into the sample vacuum chamber. (b): On the other side of the vacuum chamber opposite to the horn antenna, there is an aluminum concave mirror coaxial with the chamber. The solenoid valve nozzle (common valve series 9, diameters 0.9 mm)is installed in the center of the aluminum concave mirror, which is controlled by a special pulse driver(Parker IOTA ONE Pulse Driver). The sample gas molecular beam, after ejected into the vacuum chamber by the solenoid valve nozzle, supersonically expands and directly contacts with the chirped-pulse emitted from the horn antenna. Owing to the existence of aluminum concave mirror, the chirped-pulse microwave can be reflected to stimulate the gas molecular beam twice, which improves the utilization rate and polarization efficiency of the sample. After the polarization of the sample molecular beam, the FID signal is received by the same horn antenna and transmitted out of the cavity. (c): The FID signal is transmitted through a circulator (2→ 3) and mixed with the same single-frequency microwave signal to bring the FID frequency down. The mixed FID signal through a single-pole single-throw electronic switch (SPST, F9114A, 1~18 GHz) is then amplified by a low-noise amplifier (LNA), and digitized directly on a high-speed digital oscilloscope (Tektronix, DPO71254C). All microwave sources and trigger sources in the experiment, as well as the high-speed digital oscilloscope, are phase-locked to a 10 MHz rubidium frequency oscillator (SRS STANFORD RESEARCH SYSTEM, FS725) to provide phase stability and frequency accuracy. Vacuum degree of the sample vacuum chamber is maintained by a vacuum system based on a mechanical pump and molecular pump, which can be 10-4 Pa under experimental conditions and 10-5 Pa under non-experimental conditions.
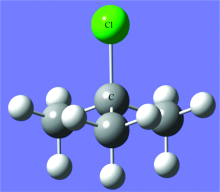
Tert-butanol (99% purity) was purchased from Aldrich and could be used without further purification. The sample was placed in a U-shaped tube at room temperature. At a backing pressure of 2.7 atm, the carrier gas (an approximate 0.5% hydrochloric acid, 99.5% neon mixture) passed through the tert-butanol sample to produce tert-butyl chloride product. The mixture was then supersonically expanded into the vacuum chamber through the nozzle with a frequency of 1 Hz and a pulse duration of 500 μ s. Nine FID signals were collected for one jet and, for the final spectrum, a total of 45 000 FID signals were averaged together in order to get better signal-to-noise ratio (SNR). Gaussian 03[9] was used to calculate the Ab initio electronic structure of tert-butyl chloride at the MP2/6-311++G(d, p) level of theory. The structural parameters of tert-butyl chloride (including atomic coordinates of the molecule, bond lengths, bond angles, dihedral angles, etc.) were obtained, as shown in Table 1 and 2 of the appendix A. The structure diagram of tert-butyl chloride calculated by Gaussian03 was shown in Figure 2, where the chlorine atomreplaces the hydrogen atom in tert-butanol and is attached to the carbon atom. The tert-butyl chloride is a symmetric top, with C3ν symmetry. Meanwhile, the calculated structural parameters were used to predict the rotational constants and inertial parameters of tert-butyl chloride and its 37Cl isotopologue, as shown in Table 1.
| Table 1 Rotational constants and inertial parameters of (CH3)3C35Cl and (CH3)3C37Cl predicted by structural parameters |
| Table 2 Observed transition frequencies (J', K, F'-J″, K, F″) of (CH3)3C35Cl and (CH3)3C37Cl with residuals from least-squares fit |
Tert-Butyl chloride can be produced by the reaction of tert-butyl alcohol with hydrogen chloride. Its microwave spectra were first studied by W. Zeil and coworkers[10, 11], by means of Stark spectroscopy. Recently, the microwave Fourier transform spectra of tert-butyl chloride were analyzed[12, 13]. In this work, the reaction mixture of tert-butyl alcohol and HCl were measured and the rotational spectra of tert-butyl chloride were assigned in 1-18 GHz region. Tert-Butyl chloride [(CH3)3C35Cl] and its 37Cl isotopologue [(CH3)3C37Cl] were observed in natural abundance. In total, 36 spectral lines were measured and assigned, among which 24 lines belong to (CH3)3C35Cl and 22 lines belong to (CH3)3C37Cl. An example of the rotational spectra of (CH3)3C35Cl and (CH3)3C37Cl observed in the experiment is shown in Figure 3. The measured transition frequencies (J', K, F'-J″, K, F″) of (CH3)3C35Cl and (CH3)3C37Cl are shown in Table 2, with uncertainty about 5 kHz from the least-squares fit.
 | Fig.3 An example of the rotational spectra of (CH3)3C35Cl and (CH3)3C37Cl measured by CP-FTMW spectrometer The (J=1→ 0, K=0) transitions of (CH3)3C35Cl and (CH3)3C37Cl are marked out |
Afterwards, the rotational spectra were analyzed by Pickett’ s SPCAT& SPFIT[14] (spectral prediction and fitting software). During the analysis, the rotational constants, centrifugal distortion and quadrupole coupling constants of (CH3)3C35Cl and (CH3)3C37Cl were fitted.The results are shown in Table 3. On the other hand, these spectral parameters were compared with the values in Ref.[15], and satisfactorily they are highly consistent.
| Table 3 Spectral parameters of (CH3)3C35Cl and (CH3)3C37Cl obtained in the experiment and relevant values in the reference |
Note: a Spectral parameters obtained in this work; b Corresponding spectral parameters shown in Ref.[15]; c The uncertainty in brackets is the standard error for the last argument unit; d Nuclear quadrupole coupling constant; e Centrifugal distortion parameter; f Root-mean-square
The performance of the broadband CP-FTMW spectrometer is proved by the products detection of achemical reaction.Owning to supersonic expansion, the rotational temperature is reduced and the spectral resolution is better, reaching 5~50 kHz. The multiply FID’ s technique is successfully used to make the sampling rate higher.The measurements of low-frequency spectra are more precise and the spectra are extended to 18 GHz. After being averaged 45 000 times, the signal-to-noise ratio of the FID signals attains about 100. As seen in Table 1, after fitted by Pickett’ s SPFIT, the differences between observed and calculated frequencies are extremely small. The root-mean-square value of (CH3)3C35Cl is only 2.1 kHz and for (CH3)3C37Cl is 2.37 kHz.
The spectroscopic constants of the reaction products, (CH3)3C35Cl and (CH3)3C37Cl, are now known accurately (see Table 3), which are highly consistent with the corresponding constants determined by a Balle-Flyg are type spectrometer in Ref.[15]. Because tert-butyl chloride is a prolate symmetric top, in spectral analysis, only the rotational constant B is fitted.In addition, the experimental value of rotational constant B is compared with its Gaussian predicted value and the difference between them is 17.632 MHz for (CH3)3C35Cl and 17.213 MHz for (CH3)3C35Cl. It can be seen that the rotational constants calculated by Gaussian is only for reference, which are a bit different from the actual experimental values.This also reflects the good performance of our microwave spectrometer.Due to more precise measurements of low-frequency spectra, nuclear quadrupole coupling constants (eqQ) are with better accuracy. Furthermore, the rs distances (where s is for substitution) are calculated as well, of which C— Cl bond length is 1.815 Å . And there is good quantitative agreement for the rs distances and values obtained in Ref.[15].
In this report, the design and performance of a 1~18 GHz CP-FTMW spectrometer were discussed. The sensitivity and relative accuracy of the spectrograph were demonstrated by the measurement of tert-butyl chloride, the chemical reaction product of tert-butyl alcohol and HCl. Spectral constants and structural parameters of two tert-butyl chloride isotopologues were reported with good accuracy.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|