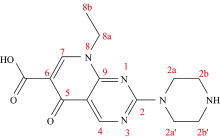
Pipemidic acid is one of an efficient quinolone antibacterial drug. Thecomplexitybetween pipemidic acid “Hpipc” withgallium(Ⅲ), germanium(Ⅳ) and silicon(Ⅳ) afforded three molecular formulas of [Ga(pipc)2(H2O)(Cl)]·4H2O, 1, [Ge(pipc)2(Cl)2]·4H2O, 2 and[Si(pipc)2(Cl)2]·4H2O, 3 complexes. These three new complexes were characterized based on elemental analysis, conductance, FTIR, UV-Vis,1HNMR and XRD spectroscopy. The pipc chelate exhibits O, O coordinated through the carbonyl (C=O) and carboxylato (COO) of both oxygen atoms. Six coordinate geometry was proposed regarding complexes of 2 and 3, so the axial position was occupied by two coordinated chlorideatoms. In vitro, the antibacterial, antifungal, and anti-cancer assessments concerning the synthesized pipc complexes were scanned. These complexes have an excellent anti-microbial activity.
The chemical nomenclature of pipemidic acid (pipc) is “ 5-oxo-5, 8-dihydropyrido[2, 3-d]pyrimidine-6-carboxylic acid” with substituted in two positions 2 and 8 by piperazinyl and ethyl moietiesas mentioned in Figure 1. The therapeutic agent of pipemidic acid as an antibiotic of quinolone derivatives is focused on urinary tract infections[1, 2]. Literature survey revealed that the Ca2+, Sr2+, Ba2+, Sn4+ complexes of the protonated pipc acid were synthesized and discussed[3], also, some of the lanthanides (La3+, Ce3+, Pr3+, Nd3+, Sm3+, Tb3+, Dy3+ and Y3+) metal ions[4] as well as copper(Ⅱ ) and managanese(Ⅱ ) pentacoordinate complexes were prepared and well characterized[5, 6].
In particular, the complexity of Ga(Ⅲ ) by multi-chelators is expected to prevent hydrolysis processes, while improving the bioavailability and permeability of the cell membrane of the compounds[7, 8].
The chemistry of coordinate silicon compounds has attracted considerable interest from many points of view such as reactivity, biological activity and structural features[9, 10]. In 1991, it was suggested that silicon play a vital role allowing the interaction between one of the many extracellular matrix proteins detected and cell surface receptors[11]. Such a role would be a mechanism by which silicon could have beneficial measures that go beyond mere connective tissue, including cardiovascular health[12], wounding[13] and the immune function, that affected by pro-inflammatory cytokines.
Taking in view the biological and medicinal importance ofpipc and the efficiency role of metal ions in biology section, herein the article is reported the synthesis of GeⅣ , GaⅢ and SiⅣ complexes ofpipc ligandin situmethanol solvent at neutralized pH. In spite of the importance of Ga(Ⅲ ) complexes as anticancer drugs, the single work reported for Ga-PPA complex[15] didn’ t discuss this effect, sothis work will give more light about this point. Complexes of pipc drug can form a new class of mineral-based bioactive compounds, moreover, they may have a tendency to reduce resistance to bacterial strains. Therefore, these complexes were investigated in vitro antibacterial activity against Gram-positive/negative bacterial strains as well as human cellular carcinoma cells (HepG-2).
The pure chemicals and reagents used in this study were purchased from “ Sigma Aldrich Chemical Corporation, St. Louis, Mo, USA” . These chemicals are summarized as: GaCl3; GeCl4; SiCl4 and pipemidic acid. The microanalytical, physical and spectroscopic techniques as well as their models which were used in the interpretations of the solid synthesized complexes can be listed as follows:
The solid pipc complexes [Ga(pipc)2(H2O)(Cl)]· 4H2O(1), Ge(pipc)2(Cl)2]· 4H2O (2) and [Si(pipc)2(Cl)2]· 4H2O (3) with yellow and white colors, respectively were synthesized by the same procedure: A mixtures of 2.0 mmol of GaCl3, GeCl4 or SiCl4 and 4.0 mmol of pipcwere stirred in 25 mL of CH3OH solvent. The mixtures were neutralized within pH=7~8 using ammonia solution, then refluxed for about three hours. The solutions of these mixtures were reduced by left them for one week. The solid yields of pipc are located within 60%~66% with higher melting point > 300 ℃. The micro analytical analyses of the calculated (Calc. ) and experimental data (Found) of the three synthesized complexes are collected in compacted as follows:
Kirby-Bauer disc diffusion modified method was employed to assessment of theantimicrobial activity of the metal complexity of pipc samples[16]. In vitro, the cytotoxicity assay of the gallium(Ⅲ ) pipc complex was performed on the human Hepatocellular carcinomacell lines(HepG-2)[17, 18]. The HepG-2 cell line was received from VACSERA Tissue Culture Unit.
The molar ratios and conductancebehaviors of gallium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) complexes of pipc drug have been studied. The elemental analyses are in well agreement with 1∶ 2 stoichiometry (metal-to-ligand) as refereed in experimental section. All the synthesizedpipc complexes are stable at room temperature, with higher melting points. These complexes are insoluble in polar solventsbut soluble in commonly organic solvents (DMF & DMSO) with gently heating. Gallium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) pipc solutions which were dissolved in DMSO showed slightly low conductance (Λ m=20~29 ohm-1· cm2· mol-1) that confirmed the non-electrolyte nature of these respected complexes[19].
An important information concerning the coordination mode of pipc ligand towards metal ions can be obtained in a first approach by comparative FTIR spectral analysis of the pipcchelate and itsgallium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) complexes (Fig.2) and the data assignments are listed in (Table 1).
| Table 1 Infrared frequencies (cm-1) bands of pipcligand and its complexes |
The FTIR spectrum of the free pipc has two distinguish bands at 3 460 and 3 378 cm-1 due to ν (OH) and ν (NH) ofthe carboxylic and imino piperazinyl groups[20]. In case of pipc complexes, it was found the broad band in this region due to the existence of the water molecules as a coordinated and/or uncoordinated modes[21]. The IR spectrum of pipc hasn’ t a ν (C=O) stretching frequency band, based on the placed of pipcmolecule in zwitterionic form[22, 23]. it is worthy mention that the proton of carboxylic group is more acidic than proton of the piperazinyl aromatic moiety. Therefore, the character nature of the carbonyl carboxylic group is absent at 1 700 c-1[24]and two new vibration bands at 1 579 and 1 362 cm-1 are presents due to ν as(COO) and ν s(COO) stretching vibration motions (see Table 1). Regarding the spectra of pipc complexes, these bands were shifted toward lower wavenumbers because of the participation of the carboxylate oxygen in the chelation process. The difference of Δ ν =[ν as(COO)-ν s(COO)] is led to recognized the coordination mode of carboxylate group. The calculated of Δ ν for GaⅢ , GeⅣ and SiⅣ pipc complexes (Table 1) located within the range of 201~219 cm-1, this confirmed that carboxylate group is acts as a monodentate chelate[25, 26]. The band with strong intensity at 1634 cm-1 is attributed to ν (C=O) of the pyridinering in case of free pipc ligand. After coordination towards metal ions, this band is shifted to lower wavenumber with changed inteosity. These assignments were supported the place of coordination between pipc chelator and central metal ions through oxygen’ s of carboxylate and carbonyl groups[27]. The new vibration bands flanked between 600~500 cm-1 are assigned to the ν (M— O) stretching vibrations[28].
UV-Vis spectrum of the pipc ligand was showed strong-to-shoulder peaks within the region of (36 496~30 864 cm-1) and (25 000~24 331 cm-1), thatassigned to π → π * and n→ π * respectively of the N— H, C=N, C=O and COOH groups. The new intense absorption band spresenceat 37 037~31 153 cm-1 is attributed to π → π * transitions of the aromatic or carboxylate organic moieties. The complexes showed bands at 28 409~25 707 cm-1 due to the L→ MCT “ charge-transfer transitions” that assigned to octahedral geometry[29].
The spectral data of 1H-NMR analysis concerningpipc free chelator and GaⅢ and SiⅣ complexes are assigned in (Table 2). By comparison between the pipcligand, [Ga(pipc)2(H2O)(Cl)]· 4H2O and [Ge(pipc)2(Cl)2]· 4H2O complexes, it was found a significant unrest on the chemical shift in the region of aromatic protons (H7 and H4) which are nearing the coordination sites[30]. The signal characteristic for piperazine proton (H-2) is exhibited at δ =8.85~8.95 ppm (unchanged), this meaning that nitrogen of piperazine ring doesn’ t sharing in the coordination process. So, the pipc ligand coordinated towards respected metal ions through both oxygen atoms of pyridone and carboxylate rings.
| Table 2 1H-NMR proton signals of the pipc ligand and its complexes |
Gallium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) pipc synthesized complexes have been assigned using micro analytical analysis, 1H-NMR, FT-IR and UV-Vis spectral studies as well as thermo gravimetric analyses. Molar ratio of prepared complexes were 1:2 of (metal:ligand). The complexes which have the general formula [Ga(pipc)2(H2O)(Cl)]· 4H2O, [Ge(pipc)2(Cl)2]· 4H2O and [Si(pipc)2(Cl)2]· 4H2O (Fig.3).
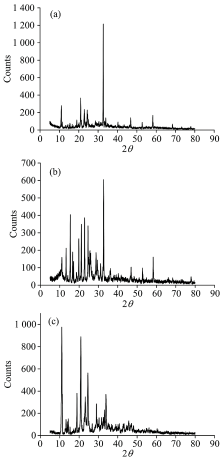
The average size of the synthesized pipc complexes for the gallium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) were determined from the Full width at half maximum of the respected XRD peaks (Fig.4) by Scherrer equation[31].
The data obtained from X-ray diffraction patterns refer to crystalline structures of the synthesized solid pipc metal complexes. Scherrer’ s formula (DRX=0.9λ /Δ cosθ ) calculation is declared to the crystal size. Where 0.9 is a term constant of the equipment, λ is the wavelength of radiation dueto the Cu Kα , peak and θ is the Bragg’ s angle. The grain size of the synthesized pipc complexes for the Ga(Ⅲ ), Ge(Ⅳ ) and Si(Ⅳ ) is < 100 nanometer.
In vitro, the antibacterial and antifungal evaluations of the pipc complexes for the gallium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) were assessed against Staphylococcus Aureus, Bacillus subtilis(G(+) bacteria) and Escherichia Coli, Pseudomonas aeruginosa(G(-) bacteria) and (Candida Albicans and Aspergillus flavus) fungi (Table 3). The results indicate that the gallium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) complexes have a significant active but less than the standard drugs Tetracycline and Amphotericin B. It is worthy mentioned that Si(Ⅳ ) complex has an significant efficient against (22 mm· mg-1) Candida Albicans fungi species rather than standard Amphotericin B drug (19 mm· mg-1). The higher activity of silicon(Ⅳ ) pipc complex against fungirevealed tothe nanosized form of this complex to make penetration during lipid membranes and blocking the sites of enzymes inthe microorganisms[32].
| Table 3 Antimicrobial assessments of GaⅢ , GeⅣ and SiⅣ pipc complexes |
The cytotoxicity (IC50) of the gallium(Ⅲ ) pipc complex was assessed against human hepatocellular carcinoma and tabulated in (Table 4). The experimental of IC50 data of the gallium(Ⅲ ) complex in vitro is equal to 123 μ g· mL-1, which can be modified in future to become a promising anticancer agent.
| Table 4 The inhibitory activityof gallium(Ⅲ ) pipc complex against HepG-2 cell line |
Acknowledgement
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|