A four new penicillinate complexes were prepared through the chemical interactions of penicillin potassium salt (Pin) with YCl3, GeCl4, WCl6 and SiCl4 metal ions. These metal complexes were characterized using spectroscopic techniques (e.g.1H-NMR, infrared, electronic UV-Vis) as well as elemental, conductivity, and magnetic measurements. The molar conductance values were highly, showing their electrolytic nature. The magnetic and electronic study strongly recommends the octahedral geometry of all penicillinate complexes. A monomeric structures of Pin complexes are proposed with octahedral coordinated metals ions. The metal ions are coordinated toward Pin as tridentate ligand through the amide and β-lactam carbonyls and a carboxylate group from penicillin. The in vitro antimicrobial activity of all the complexes, at concentrations in μg·mL-1, was screened against four bacterial pathogens, namely, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus, and two kinds of fungi Aspergillus flavus and Candida albicans showed better activity compared to parent drug and control drug. The anti-cancer inhibition of the tungsten(Ⅵ) complex was assessed against the human hepato cellular carcinoma (HepG-2) tumor cell line with IC50 value is 646 μg·mL-1.
The β -lactam pharmacy family is one of an important popular antibiotic drugs which has a broad spectrum. The penicillin antibiotic has potent bactericidal activity against many gram positive and gram negative bacterial pathogens[1, 2]. It takes action against bacteria by preventing them from forming the cell wall and stopping them from growing. In medical science, it has important application for the treatment of bronchitis, ear infection, pneumonia, throat infections, tonsillitis, typhoid, and urinary tract infections. In combination with other antibiotics, it bears potential applications for the successful treatment of many pathogenic infections.
The first study addressing metal complexes of penicillins presented at 1957[3]. The interaction of potassium salts of benzylpenicillin (Bzp) and Cu2+ ions in aqueous solution was studied by an ion exchange method[3]. Cu(Bzp)+ and Cu(Bzp)2 complexes were identified and their formation constants. It was suggested that these complexes were chelates because of coordination of the ligand (the Bzp- anion) via the O atom of the carboxylate group and the N atom of the β -lactam group. Spectrophotometric studies of the interaction of phenoxymethylpenicillin (Fmp)- with Cu(Ⅱ ) and Co(Ⅱ )[4, 5] demonstrated formation of complexes ML and ML2. Coordination of Fmp-via the carboxylate group and the β -lactam N atom was suggested. Computer modeling of the structure of the complex of Zn(Ⅱ ) with the anion of a hypothetical methylpenicillin in aqueous solution using the AM1 and PM3 semiempirical methods predicted two possible variants of ligand coordination: via the O atom of the carboxylate group and the N atom of the β -lactam group or via the two O atoms of the carboxylate group. Studies reported in [6] described solid bi-ligand complexes of Bzp- with Ni(Ⅱ ), Zn(Ⅱ ), Cd(Ⅱ ), Fe(Ⅲ ), and La(Ⅲ ) from aqueous solutions. Results from elemental analysis of reaction products were presented, along with IR and EPR spectroscopy results. These experiments led to the conclusion that in Fe(Ⅲ ) and La(Ⅲ ) complexes, the ligands were coordinated via the O atoms of the carboxylate and amide groups, while coordinations in Ni(Ⅱ ), Zn(Ⅱ ), and Cd(Ⅱ ) complexes also involve the O atoms of the β -lactam group. Solid complexes of Bzp- with Fe(Ⅱ ) were prepared and investigated by IR spectroscopy in [7]. These investigations showed that Bzp- is coordinated via the carboxylate group and the β -lactam N atom. The reaction of Ni(Ⅱ ), Zn(Ⅱ ), Cd(Ⅱ ), Fe(Ⅲ ) and La(Ⅲ ) ions with sodium penicillinate at room temperature have been investigated[8]. Two types of complexes has been isolated as M(pen)2· nH2O (M=Ni(Ⅱ ), Zn(Ⅱ ), Cd(Ⅱ ); n=3, 4) and M(pen)2· Cl· nH2O (M=Fe(Ⅲ ), La(Ⅲ ); n=2). Two novel platinum(Ⅱ ) complexes were obtained from the reaction of K2PtCl4 with penicillin V and penicillin G. The structure of these complexes was determined by using IR and NMR spectroscopy. A new, five-membered ring results from the chelation of the Pt2+ ion by the amide and thioether groups of the penicillin moiety[8]. The bare deprotonated penicillin G, and its complexes with the Ba2+, Zn2+ and Cd2+ ions, have been formed by electrospray ionization and studied by infrared multiple photon dissociation spectroscopy using Fourier-transform ion cyclotron resonance mass spectrometry. The Ba(Penicillin-H)+ ion is assigned as the simple complex with barium chelated by all three carbonyl oxygens, along with a cation-π interaction with the phenyl ring. The Zn(Penicillin-H)+ spectrum is consistent with simple complexation in a similar tridentate conformation. The Cd(Penicillin-H)+ complex probably has the simple tridentate conformation with strong cation-π interaction[9].
In the present paper, we have focused on the synthesis of new penicillinate complexes of yttrium(Ⅲ ), germanium(Ⅳ ), tungsten(Ⅵ ) and silicon(Ⅳ ) metal ions by the chemical interactions of penicillin potassium salt and its four metal chlorides salts. The coordination behavior of the ligand towards metal ions was fully investigated by various spectral techniques. The geometry of the complexes was confirmed by molar conductance, electronic and magnetic analyses. In continuation of our antibiotic research, we have also evaluated the antibacterial efficacy of penicillin ligand and its metal complexes against bacteria (Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus) and fungi (Aspergillus flavus and Candida albicans) as well as human hepato cellular carcinoma (HepG-2) tumor cell line.
Penicillin potassium salt drug (Fig.1), YCl3· 6H2O, GeCl4, WCl6 and SiCl4 salts (Sigma Aldrich Chemical Corporation, St. Louis, Mo, USA) and all the other chemicals and solvents were of analytical grade and used without further purifications.
[Y(Pin)2]· Cl· 6H2O complex (1): A mixture of 1.0 mmol of YCl3· 6H2O and 2.0 mmol of penicillin potassium salt were mixed in methanol (25 mL), then refluxed for 2 hrs, white precipitate is formed with yield 74%. Dec. temp. 260 ℃. Anal.: (Calc.) Found, %: C, (42.74) 42.70; H; (5.16) 5.09; N, (6.23) 6.11; Y, (9.89) 9.76. Λ M=71 Ω -1· cm2· mol-1. [Ge(Pin)2]· 2Cl· 2H2O complex (2): A mixture of 1.0 mmol of GeCl4 and 2.0 mmol of penicillin potassium salt were mixed in methanol (25 mL), then refluxed for 2 hrs, white precipitate is formed with yield 67%. Dec. temp. 275 ℃. Anal.: (Calc.) Found, %: C, (45.41) 45.39; H; (4.53) 4.42; N, (6.62) 6.43; Ge, (8.58) 8.41. Λ M=112 Ω -1· cm2· mol-1. [W(Pin)2]· 4Cl complex (3): A mixture of 1.0 mmol of WCl6 and 2.0 mmol of penicillin potassium salt were mixed in methanol (25 mL), then refluxed for 2 hrs, blue precipitate is formed with yield 65%. Dec. temp. 310 ℃. Anal.: (Calc.) Found, %: C, (38.73) 38.54; H; (3.45) 3.39; N, (5.65) 5.42; W, (18.52) 18.39. Λ M=239 Ω -1· cm2· mol-1. [Si(Pin)2]· 2Cl· 2H2O complex (4): A mixture of 1.0 mmol of SiCl4 and 2.0 mmol of penicillin potassium salt were mixed in methanol (25 mL), then refluxed for 2 hrs, white precipitate is formed with yield 61%. Dec. temp. 200 ℃. Anal.: (Calc.) Found, %: C, (47.94) 47.30; H; (4.78) 4.60; N, (6.99) 6.87; Si, (3.50) 3.44. Λ M=109 Ω -1· cm2· mol-1.
The elemental analyses of % carbon, % hydrogen, and % nitrogen contents were performed using a Perkin Elmer CHN 2400 (USA). The percentage of respected metal ions were estimated gravimetrically. The molar conductivities of freshly prepared 1.0× 10-3 mol· cm-3 dimethylsulfoxide (DMSO) solutions were measured for the dissolved compounds using Jenway 4010 conductivity meter. The infrared spectra were recorded on Bruker FTIR Spectrophotometer (4 000~400 cm-1). The UV-Vis absorption spectra were recorded in DMSO solvent within 800~200 nm range using a UV2 Unicam UV/Vis Spectrophotometer fitted with a quartz cell of 1.0 cm path length. Magnetic moments were calculated using the Magnetic Susceptibility Balance, Sherwood Scientific, Cambridge Science Park, Cambridge, England, at Temp 25 ℃. The 1H-NMR spectra were recorded on Varian Mercury VX-300 NMR spectrometer. 1H spectra were run at 300 MHz spectra in deuterated (DMSO-d6). Chemical shifts are quoted in δ and were related to that of the solvents.
Antimicrobial activity of the tested samples was determined using a modified Kirby-Bauer disc diffusion method[10]. Briefly, 100 μ L of the tested bacteria (G(+) (Escherichia coli and Pseudomonas aeruginosa), G(-) (Bacillus subtilis and Staphylococcus aureus)/fungi (Aspergillus flavus and Candida albicans) were grown in 10 mL of fresh media until they reached a count of approximately 108 cells· mL-1 for bacteria or 105 cells· mL-1 for fungi[11]. 100 μ L of microbial suspension was spread onto agar plates corresponding to the broth in which they were maintained. Isolated colonies of each organism that might be playing a pathogenic role should be selected from primary agar plates and tested for susceptibility by disc diffusion method[12, 13].
The mammalian cell lines: HepG-2 cells (human Hepatocellular carcinoma) was obtained from VACSERA Tissue Culture Unit. Chemicals used: dimethyl sulfoxide (DMSO), crystal violet and trypan blue dye were purchased from Sigma (St. Louis, Mo., USA). Fetal Bovine serum, DMEM, RPMI-1640, HEPES buffer solution, L-glutamine, gentamycin and 0.25% Trypsin-EDTA were purchased from Lonza. Crystal violet stain (1%): It composed of 0.5% (w/v) crystal violet and 50% methanol then made up to volume with ddH2O and filtered through a Whatmann No.1 filter paper. Cell line Propagation: The cells were propagated in Dulbecco’ s modified Eagle’ s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine, HEPES buffer and 50 μ g· mL-1 gentamycin. All cells were maintained at 37 ℃ in a humidified atmosphere with 5% CO2 and were subcultured two times a week. Cytotoxicity evaluation using viability assay[14, 15]: For cytotoxicity assay, the cells were seeded in 96-well plate at a cell concentration of 1× 104 cells per well in 100 μ L of growth medium. Fresh medium containing different concentrations of the test sample was added after 24 h of seeding. Serial two-fold dilutions of the tested chemical compound were added to confluent cell monolayers dispensed into 96-well, flat-bottomed microtiter plates (Falcon, NJ, USA) using a multichannel pipette. The microtiter plates were incubated at 37 ℃ in a humidified incubator with 5% CO2 for a period of 48 h. Three wells were used for each concentration of the test sample. Control cells were incubated without test sample and with or without DMSO. The little percentage of DMSO present in the wells (maximal 0.1%) was found not to affect the experiment. After incubation of the cells for at 37 ℃, various concentrations of sample were added, and the incubation was continued for 24 h and viable cells yield was determined by a colorimetric method. In brief, after the end of the incubation period, media were aspirated and the crystal violet solution (1%) was added to each well for at least 30 minutes. The stain was removed and the plates were rinsed using tap water until all excess stain is removed. Glacial acetic acid (30%) was then added to all wells and mixed thoroughly, and then the absorbance of the plates were measured after gently shaken on Microplate reader (TECAN, Inc.), using a test wavelength of 490 nm. All results were corrected for background absorbance detected in wells without added stain. Treated samples were compared with the cell control in the absence of the tested compounds. All experiments were carried out in triplicate. The cell cytotoxic effect of each tested compound was calculated. The optical density was measured with the microplate reader (SunRise, TECAN, Inc, USA) to determine the number of viable cells and the percentage of viability was calculated as [1-(ODt/ODc)]× 100% where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells. The relation between surviving cells and drug concentration is plotted to get the survival curve of each tumor cell line after treatment with the specified compound. The 50% inhibitory concentration (IC50), the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphic plots of the dose response curve for each conc. using Graphpad Prism software (San Diego, CA. USA).
The physical properties and the microanalytical data of the penicillinate metal complexes (1— 4) are summarized in the experimental section. The analytical results show (1:2) metal:ligand ratio, that is, ML2 formula. The color of all complexes is white but tungsten(Ⅵ ) complex has a blue color with change from ligand to metal complexes, that is support of metal ligand interaction which is further strengthened by measurement of conductivity data. The ligand (Pin) in potassium salt form was soluble in water and methanol. The complexes were slightly soluble in DMSO and DMF with gently heating. The yttrium(Ⅲ ) complex 1, germanium(Ⅳ ) complex 2, silicon(Ⅳ ) complex 4 were found to be hydrated but the tungsten(Ⅵ ) complexes has an anhydrous form. The suggested molecular formulae of the ligand (Pin) and metal complexes (1— 4) have been accomplished by microanalytical results incorporation with different essential spectroscopic techniques. The experimental molar conductivity data of metal complexes was found in the range of 71~239 ohm-1· cm2· mol-1 and suggests their electrolytic behavior[16].
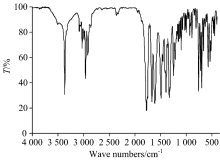
FTIR spectra of free Pen ligand and its four Y(Ⅲ ), Ge(Ⅳ ), W(Ⅵ ) and Si(Ⅳ ) penicillinate complexes are presence in Fig. 2(a, b) and the distinguish infrared wavenumbers of function groups are assigned within the Mid infrared region 4 000~400 cm-1 and summarized in Table 1. The must characteristic vibration bands of Pin ligand are specialized to β -lactam, carboxylate, amido and dimethylthiazolidine groups.
In case of free Pin ligand [Fig.2(a)], the stretching vibration of ν (C— H) aromatic group is exhibit at 3 084 and 3 029 cm-1 [17]. Out-of-plane bend of the C— H bands in the free Pin ligand are presence within 1 000~650 cm-1, these frequencies are existed at the low wavenumbers after complexity. In-plane bend frequencies are existed in 1 300~1 000 cm-1 region. The v(C=C) skeletal stretching vibrations are placed within 1 650~1 450 cm-1 region[18]. The stretching vibration of ν (NH) amido group is existed at 3 369 cm-1. Stretching vibration bands at 1 776 cm-1 is assigned to v(C=O) of β -lactam. A very strong band noticed at 1 670 cm-1 is assigned to stretching vibration of v(C=O) of the amido group. Two distinguish bands at 1 613 and 1 395 cm-1 are assigned to asymmetric and symmetric stretching vibrations of COO carboxylate group, respectively.
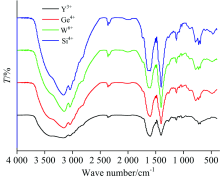
In comparable between free Pin drug ligand and their yttrium(Ⅲ ), germanium(Ⅳ ), tungsten(Ⅵ ) and silicon(Ⅳ ) complexes, there are some differences in frequencies and intenisties due to chelation process between Pin potassium salt toward respected metal ions. All penicillinate complexes have the same pathway of coordination via oxygen atoms of β -lactam, amido and carboxylate groups. In the Pin complexes, the stretching vibration band of carbonyl group v(C=O) β -lactam ring is shifted to lower frequencies or absence due to involvement in chelation toward central metal ions. In case of tungsten(Ⅵ ) and silicon(Ⅳ ) complexes, this band was shifted from 1 776 cm-1 (free Pin ligand) to 1 750~1 737 cm-1, respectively, but the yttrium(Ⅲ ) and germanium(Ⅳ ) complexes this frequency was absent. The stretching vibration band of carbonyl v(C=O) of the amido group is absence in case of metal complexes suggesting that chelation of ligand occurs through oxygen atom of amido group. The carboxylate group is able to coordinate to metal ions by different mode of chelations[19]. The carboxylate group coordinated toward metal ion as a unidentate when the difference wavenumbers of carboxylate groups, Δ ν =(ν asCOO--ν sCOO-) is > 200 cm-1. The Δ ν is less than < 200 cm-1, when the carboxylate group acts as bidentate mode[20]. When Δ ν is larger than chelated ions and nearly the same as observed for ionic compounds, the carboxylate group acts as bridged bidentate. From the study of different coordination modes of carboxylate group, it was confirmed that the carboxylate group act as unidentate chelation, because of the difference wavenumbers between (ν asCOO-ν sCOO) have data between 200~258 cm-1. The hydrated Y(Ⅲ ), Ge(Ⅳ ) and Si(Ⅳ ) penicillinate complexes have a shoulder vibration bands at ~3 400 cm-1, which was assigned to ν (OH) hydrated water molecules. From Far-IR (Fig.3) as well as Mid-IR, the assignments of three different vibration bands of ν (M— O); (oxygen’ s of β -lactam, amido, carboxylate groups) are existed within the 400~600 cm-1 range[21, 22, 23].
| Table 1 Assignments of the FTIR frequencies (cm-1) of penicillin potassium salt and their metal complexes |
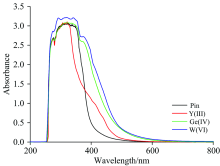
The UV-Visible spectra of free potassium penicillinate and the Y(Ⅲ ), Ge(Ⅳ ), W(Ⅵ ) and Si(Ⅳ ) complexes in DMSO solvent (Fig.3) have two maxima interplay absorption bands diverse regions at 274~277 and 318~338 nm, attributed to intraligand excitation[7]. The diamagnetic complexes of Y(Ⅲ ), Ge(Ⅳ ) and Si(Ⅳ ) also display intense charge transfer transitions in the visible within 373~410 nm region. The tungsten(Ⅵ ) complex also exhibits a weak shoulder absorption band at 376 nm, that emerge from a d— d transition. The effective magnetic moments (μ eff) of yttrium(Ⅲ ), germanium(Ⅳ ) and silicon(Ⅳ ) penicillinate complexes were calculated at room temperature, the resulted data confirm that these mentioned complexes have a diamagnetic nature with coordination mode as octahedral geometry[24]. The magnetic moment of the tungsten(Ⅵ ) complex is within the range of values previously reported for tungsten in case of the six oxidation state[25, 26].
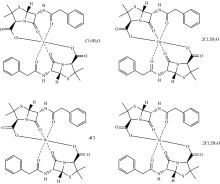
The 1H-NMR spectra of penicillinate potassium ligand and germanium(Ⅳ ) complex in DMSO-d6 solvent were scanned. Penicillinate potassium druge ligand is assigned as: δ (ppm) 1.441 and 1.555 (6H, 2CH3 group of dimethyl thiazolidine ring), 3.359 (2H; CH2 group), 3.540 (1H; thiazolidine ring), 3.843 (H; NH of amide group), 5.310 (2H; β -lactam), 7.268 and 8.690 (5H; benzene ring). Germanium(Ⅳ ) penicillinate complex is assigned as: δ (ppm) 0.941~2.082 (6H, of dimethyl group of thiazolidine ring), 3.425 (2H; CH2 group), 3.594 (1H; thiazolidine ring), 3.655 (H; NH of amide group), 5.593 (2H; β -lactam), 7.244 and 8.621 (5H; benzene ring). In the neutral case, penicillinate potassium ligand remains deprotonated and coordinates to Y(Ⅲ ), Ge(Ⅳ ), W(Ⅵ ) and Si(Ⅳ ) ions as a tridentate chelate using the amide and the β -lactam carbonyl groups and the carboxylate oxygen atom as speculated in this study (Fig.4) by spectroscopic analyses. The thiazolidine-S atom and the amide-N atom are unlikely to coordinate in this case, due to angle strain[7].
Antimicrobial assessments of the penicillinate potassium free drug and Y(Ⅲ ), Ge(Ⅳ ), W(Ⅵ ) and Si(Ⅳ ) complexes have been carry out in vitro against bacteria (G(+) (Escherichia coli and Pseudomonas aeruginosa), G(-) (Bacillus subtilis and Staphylococcus aureus) and fungi (Aspergillus flavus and Candida albicans). Inhibition zones diameters of the test complexes are listed in Table 2. Yttrium(Ⅴ ) complex has an effective inhibition against Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans rather than free Pin free drug. Germanium(Ⅳ ) complex has an effective inhibition against Bacillus subtilis, Escherichia coli, Aspergillus flavus and Candida albicans rather than free Pin drug. Tungsten(Ⅵ ) complex has an effective inhibition against Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Aspergillus flavus and Candida albicans rather than free Pin drug. Silicon(Ⅳ ) complex has an effective inhibition against Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans rather than free Pin drug. The highly efficiency of antimicrobial activity of the penicillinate complexes can be assigned to the ability of penetration through the lipid membranes and blocking of the metal biding sites in the enzymes of microorganisms. A possible mode of action is that the polarity of the metal ion is reduced because of partial sharing of its positive charge with the donor groups and possible π -electron delocalization over whole chelate ring[27].
| Table 2 Inhibition zone diameter (mm· mg-1 sample) of Pin free and their complexes against some kind of bacteria and fungi |
In vitro cytotoxicity assessments of the tungsten(Ⅵ ) complex was carried out on the human hepatocellular carcinoma (HepG-2) tumor cell line. The outcome data assessed based on the calculation of inhibitory concentration of IC50, the data was listed in Table 3. The IC50 of this complex is 646 μ g· mL-1 against HepG-2 cell line.
| Table 3 The inhibitory activities against HepG-2 cell lines for the tungsten(Ⅵ ) complex |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|