e-mail: sarsarvikas@gmail.com
The aim of this study to focused on bioinspired synthesis of silver nanoparticles (AgNPs) as a viable alternative to eradicate the existing physicochemical processes. In this context, the bioinspired AgNPs were synthesized using leaf extract of M. indica. Optimization of the experimental conditions for the rapid and high yield of AgNPs in minimum investment of time and expense have been carried out along with their antibacterial efficacy evaluated. For this reason, the variation of parameters like the concentration of the silver precursors, reducing agent, time, pH, and temperature of synthesis were realized. Synthesized AgNPs were characterized by UV-Visible spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and transmission electron microscopy (TEM) techniques. UV-Visible spectra gave surface plasmon resonance (SPR) at 440 nm for AgNPs. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) techniques were further confirmed the synthesis and crystalline nature of AgNPs respectively. Transmission electron microscopy (TEM) observed spherical shapes of synthesized AgNPs within range 5~20 nm. The results of the current study indicate that optimization process play a pivotal role in the AgNPs synthesis and biogenic synthesized AgNPs might be used against bacterial pathogens.
From the last decade, nanotechnology has revolutionized the entire biological sciences which lead the commercialization of nano based products such bactericides, fungicides, pesticides, anticancer etc. AgNPs have large number of applications in field of catalysis (Kamat 2002), plasmonics (Maier et al. 2001), optoelectronics (Mirkin et al. 1996), biological sensor (Gracis et al. 2002) antimicrobial activities (Shahverdi et al. 2007; Sarsar et al. 2014a), DNA sequencing (Cao et al. 2001), and surface-enhanced Raman scattering (SERS) (Matejka et al. 1992). Instead of these potent applications, AgNPs has delivered solution to various problems like climate change and pollution control (Shan et al. 2009), clean water technology (Mccuen 2010), energy generation (Zach et al. 2006), information storage (Sandhu., 2008) and biomedical applications (Caruthers et al. 2007). Synthesis of AgNPs has great revolution in the field of nanotechnology in the last ten years and proves its potentials by new and varied applications. AgNPs are synthesized by various physical and chemical methods. Chemical methods include silver salt precursor that is reduce during chemical reaction (Hullman., 2009). Physical methods such as lithography and laser ablation (Amendola et al. 2006). Both physical and chemical methods takes lots of time, expensive and have toxic effect on environment (Tolaymat et al. 2010). Development of eco- friendly and sustainable techniques for the synthesis of AgNPs is a challenge. Reports studied showed that it can be synthesized by bacteria (Reddy et al. 2010, fungi (Sarsar et al. 2014b) and plants (Sarsar et al. 2014c). It is found that plants are more suitable than bacteria and fungi for the extracellular synthesis of AgNPs as they are easily available, safe to handle, eliminating the elaborate process of maintaining microbe cultures, and possess a broad variability of metabolites may aid in reduction. Synthesis of AgNPs using several plant extracts, particularly Psidium guajava, Azadirachta indica, mangosteen, Acalypha indica, Rosa damascene, mangrove, Citrus limon etc. has been already documented in various approaches (Sarsar et al. 2014c; Shankar et al. 2004; Veerasamy et al. 2011; Krishanraj et al. 2010; Tripathy et al. 2010; Prathna et al. 2011). Considering the significance of bio-based fabricated nanomaterials, the present study was designed to undertake the extracellular biosynthesis of silver nanoparticles by using the leaf extract of M. indica. The synthesized silver nano particles have been characterized by various techniques. During this investigation we have emphasize on the various significant parameters for optimization for maximum yield of silver nanoparticles.
Leaves of M. indica were collected from the botanical garden CSSRI, Karnal, India. Silver nitrate was purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India. Antibacterial efficacy of AgNPs tested against Aeromonas hydrophila (MTCC-1739), Bacillus cereus (MTCC-1305), Staphylococcus aureus (MTCC-3160) Salmonella typhimurium (MTCC-1253), Enterobacter aerogenes (MTCC-2823) and Micrococcus luteus (MTCC-1809) were procured from Institute of Microbial Technology (IMTECH), Chandigarh, India.
The fresh leaves were thoroughly cleaned under running tap water followed by double distilled water. The leaves were dried at room temperature for 2~3 days. Ten gram of the dried leaves were cut sliced in to small pieces using a sharp knife. Then small pieces of leaves stirred using magnetic stirrer in 100 mL of double deionized water at 60 ℃ in 250 mL Erlenmeyer flask for 30 min. Then this solution was filtered through Whatman No.1 filter paper (pore size 25 μ m). For the reduction of silver ions, different concentrations of leaf extract was mixed to different concentration of aqueous solution of AgNO3 in 250 mL Erlenmeyer flask and incubated at different temperature. A control setup was also maintained without M. indica extract.
Various parameters were optimized for synthesis of AgNPs including concentration of silver nitrate, concentration ratio of leaf extract and silver nitrate, time, temperature and pH. Optimized condition for synthesis of AgNPs was monitored by using UV-Visible spectrophotometer (Systronic-115, India) with a resolution of 1.0 nm between 300 to 600 nm.
The effect of the silver salt on AgNPs synthesis was monitored by varying the concentration of AgNO3 1 to 5 mmol· L-1 (1, 2, 3, 4 and 5 mmol· L-1) and the optimized concentration of metal ion. Concentration ratio of leaf extract and silver nitrate was varied for the production of AgNPs by using different ratio of leaf extract and silver nitrate. (1:9, 2:8, 3:7, 4:6, and 5:5). The temperature of the reaction was optimized by incubation at 5, 15, 25, 35 and 45℃ respectively, where the reaction temperature was maintained using water bath. Ideal time for synthesis of AgNPs was monitored by measuring the UV-visible spectrum of the reaction medium at 5, 10, 15, 20 and 25 minutes. Optimum pH was selected by incubating the reaction mixture 2, 5, 7, 9 and 11. The pH was maintained with help of 0.1 N HCl and 0.1 N NaOH.
Synthesized AgNPs under optimized conditions was purified by repeated centrifugation at 10 000 rpm for 30 min and redispersed the pellet in sterile deionized water and further characterized using FTIR, XRD and TEM.
Fourier transform infrared spectroscopy analysis (FTIR) of synthesized AgNPs was recorded with Thermo Scientific Nicolet iS50- India) with resolution at 4.000 from 400~4 000 nm.
X-ray diffraction measurements (XRD) patterns of synthesized AgNPs was recorded by using X’ Pert Pro PANalytical X-ray diffractometer instrument. XRD was operated at a voltage of 40 kV and a current of 40 mA with Cu Kα radiation. The crystallite domain size was calculated from the width of the XRD peaks using the Scherrer formula.
Where D is the average crystallite size perpendicular to the reflecting planes, λ is the wavelength, β is the full width at half maximum, and θ is the diffraction angle.
Transmission electron microscopy (TEM) employed for the size and shape of synthesized AgNPs. TEM carried out by using H-7500 electron microscope (Hitachi, Japan) at an accelerating voltage of 120 kV. For transmission electron microscope (TEM) measurements, a drop of diluted sample of AgNPs suspension was placed on the carbon coated copper grids and allowed water to evaporate.
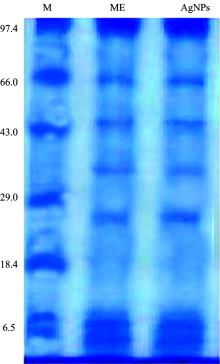
SDS Electrophoresis carried out to observe the associations of proteins in synthesis and stability of AgNPs. Both purified AgNPs suspensions and M. indica extract (ME) were precipitated by adding solid ammonium sulphate 75%w/v). For desalting, the proteins were washed with sodium phosphate buffer (0.05 mol· L-1, pH 7.0) at least three times. Afterwards, the samples were dialyzed overnight with the same sodium phosphate buffer (0.05 mol· L-1, pH 7.0). Then protein was examined by running the samples in SDS-PAGE as per standard protocol. The sample was dissolved in sample buffer and heated the sample at 80 ℃ for 10 min. The sample was cooled and mixed with bromophenol blue and loaded in the gel. After running of protein sample in the gel, the gel was stained with Coomassie brilliant blue and observed.
Determination of antibacterial efficacy were determined using the agar well diffusion method. Antibacterial activity of bacterial cultures was carried on Muller-Hinton Agar plates using 20 μ L nanoparticles suspension.
Statistical analysis each experiment was carried out in triplicate and results are presented as the mean± Standard Deviation (SD) in the respective figures.
On exposure to colorless AgNO3 solutions, M. indica extract formed dark brown colored solutions that indicated the formation of silver nanoparticles [Fig.1(a)]. UV-Vis spectrum of synthesized AgNPs shows a characteristics peak at 440 nm. Generation of brown color due to the surface plasmon resonance exhibited by the silver nanoparticles (Vaidyanathan et al. 2010).
From past few year, lots of literatures regarding synthesis of AgNPs but very few related to optimization. The present study demonstrated the influence of various parameters dependent synthesis of AgNPs from M. indica leaf extract. 1 mmol· L-1 concentration of silver nitrate gave sharp peak at 440 nm in UV-Vis spectrum, whereas the peak got shifted at 2, 3, 4, and 5 mmol· L-1 concentrations [Fig.1(b)]. Krishnaraj et al reported that concentration of 1 mmol· L-1 of silver nitrate was sufficient for synthesis of AgNPs using Acalypha indica leaf extract (Krishanraj et al. 2010). Our study have similar results using different concentration of silver nitrate from 1 to 5 mmol· L-1. By gradual increase in concentration of silver nitrate up to 5 mmol· L-1, the production decreased. It might be interpreted that the optimum concentration of silver nitrate is 1 mmol· L-1.
The ratio of 1 mL of M. indica leaf extract along with 9 mL of silver nitrate showed characteristic peak at 420 nm, whereas the peak got shifted at concentration ratio of 2:8, 3:7, and 4:6 and 5:5 [Fig.1(c)]. The ratio of silver nitrate solution (1 mmol· L-1) and the leaves extract was changed to examine the optimum composition uo maximize the yield of AgNPs. Govindaraju et al suggested that 1 mL of extract in 9 mL of AgNO3 much sufficient for converting all silver ions to silver nanoparticles (Govindaraju et al. 2010). The similar results from our study corroborate with the earlier reported study.
UV-Visible spectrum showed strong and characteristic absorption peak at 440 nm after 25 minutes, [Fig.1(d)]. As the time of reaction increases, more AgNPs formed. The optimum time required for the completion of reaction from our study was 25 min correlated with the earlier reported study (Venkatesan et al. 2014).
UV-Visible spectra recorded at different temperature showed a sharp peak observed at 25 ℃[Fig.1(e)]. Sarsar et al (2014c) have reported the effect of temperature on synthesis of AgNPs by using leaf extract of Psidium guajava. The author’ s found that temperature of 25 ℃ showed maximum productivity of silver nanoparticles. The reduction of silver ions to silver nanoparticles dependence on the temperature could be associated with the metabolites stability exists in the leaf extract during current investigation. There is A sharp peak observed at pH 7 while no sharp peak observed at acidic pH (2 and 5) as well as basic pH (9 and 11) [Fig.1(f)]. Leaf extract of M. indica stable at neutral pH while at higher pH and lower pH, the various proteins and metabolites present in extract did not show any functional activity for synthesis of AgNPs. Our results correlate with the reports of Veerasamy et al (2011) wherein the AgNPs synthesis carried using mangosteen leaf extract. This study clearly showed no alteration in the peak at 420 nm even after 2 months of incubation period, indicating strong stability of biosynthesized silver nanoparticles. Therefore, it is clear that the optimization process played a pivotal role in the particles stability and aggregation.
FTIR measurements of synthesized AgNPs in suspension carried out to examine the possible interactions between silver and biomolecules which responsible for the both synthesis and stabilization. FTIR spectrum showed three distinct peaks, 462.26, 1 640.82 and 3 320.52 cm-1(Fig.2). Absorbance bands were observed at 462.26, 1 640.82 and 3 320.52 cm-1. Band at 3 320.52 cm-1 refers to the stretching vibrations of primary amines while band at 1 640.82 cm-1 which is attributed to the C=O stretching due to the carboxyl content in the leaf extract of M. indica, which might reduce the silver ion to silver nanoparticles while the peak at 462.26 cm-1 is the fingerprint. The carbonyl groups of amino acid residues and peptides have strong ability to bind to silver. Similar results reported from earlier study (Sarsar et al. 2014; Veerasamy et al. 2011).
Formation and quality of the synthesized silver nanoparticles has been deduced from the XRD spectrum. XRD patterns of the synthesized AgNPs showed in the Fig.3. XRD spectra clearly indicated the pure silver crystalline nature. The obtained data was matched with the database of Joint Committee on Powder Diffraction Standards (JCPDS file No.04-0783). XRD spectrum evident that the silver particles formed were in the nano size and the peaks at 2θ values of 36.129 corresponding to (111) plane for silver, respectively. The full width at half maximum (FWHM) values measured for 111 plane of reflection were used with the Debye-Scherrer’ s equation d=0.9λ /β cosθ . Our results indicated that the synthesized silver nanoparticles by Penicillium atramentosum KM was in the form of nanocrystals. Our study accordance with the earlier reports (Tripathy et al. 2010; Prathna et al. 2011).
Electron microscopic imagining allows measuring the size and shape of the silver nanoparticles formed. TEM images of the synthesized AgNPs represented in Fig.4. TEM images of the synthesized AgNPs have shown spherical shape and large distribution of sizes in the range of 5~20 nm. Spherical shape and size of AgNPs in range of 5~20 nm have been reported using leaf extract of Azadirachta indica and Rosa damascena (Tripathy et al. 2010; Venkantesan et al. 2014).
SDS-PAGE electrophoretic runs electrophoresis showed proteins of 99, 65, 44, 25 and 6 kDa in both M. indica extract (ME) and AgNPs suspensions (Fig.5). Similar pattern of protein bands observed in both M. indica extract (ME) and AgNPs suspensions indicated that proteins present in extract remained same while the formation of the AgNPs. Furthermore, these proteins stabilized the synthesized AgNPs by capping to their surfaces (Rodrigues et al. 2013).
Synthesized AgNPs show significant antimicrobial activity against bacterial pathogens B. cereus, S. aureus, S. typhimurium, A. hydrophila, E. aerogenes and M. luteus (Fig.6). Synthesized silver nanoparticles showed highest antimicrobial activity against and S. aureus and A. hydrophila showed a zone of inhibition of 22 and 21 mm. AgNPs in nano scale delivers a tremendously large surface area for better contact with bacteria that enhances permeability of the cell membranes and results in cell death (Shahverdi et al. 2007; Sarsar et al. 2014a).
The first author wishes to thank University Grant Commission, New Delhi for providing the Senior Research fellowship for the completion of his PhD. Authors are thankful to SAIF, Punjab University Chandigarh for providing TEM, XRD and FTIR facility.
Conflict of Interest: No conflict of interest declared.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|