以铈箔为原料, 采用阳极氧化法和热处理法制备多孔的CeO2膜。 将阳极氧化铈膜分别在400, 500和800 ℃下进行热处理, 分别研究阳极氧化铈膜的晶体结构、 组成和表面形貌, 分别研究多孔的CeO2膜红外光谱特征吸收和热膨胀性能。 阳极氧化铈膜是Ce(OH)3, CeF3, Ce2O3, CeO2和Ce的混合膜, 并吸附水和乙二醇, 其中Ce(OH)3, CeF3, Ce2O3分别为六方晶型结构, CeO2和Ce分别为立方晶型结构。 阳极氧化铈膜中的Ce(OH)3, Ce2O3和Ce分别在400和500 ℃进行热处理时可能分别转变为CeO2, 分别在400和500 ℃热处理后的膜为CeF3和CeO2的混合膜。 阳极氧化铈膜中的Ce(OH)3, CeF3, Ce2O3和Ce在800 ℃进行热处理时可能分别转变为CeO2, 在800 ℃热处理后的膜为CeO2膜。 该CeO2膜是多孔的膜, 且孔为直孔, 在1 600~4 000 cm-1范围内具有强吸收。 该CeO2膜在170~900 ℃范围内热膨胀系数变化不大, 该膜的热稳定性较好。
The porous CeO2 films were prepared with anodization and heat treating method with cerium foils as raw materials. The anodic cerium oxide films were heat treated at 400, 500 and 800 ℃ respectively. The crystal structures, compositions, surface morphologies of the cerium dioxide films were investigated, respectively. The properties of IR characteristic absorption and thermal expansion of the porous CeO2 film were investigated respectively. The anodic cerium oxide film is composed of Ce(OH)3, CeF3, CeO2, Ce2O3 and Ce, and the crystal structures of Ce(OH)3, CeF3, Ce2O3 and CeO2, Ce are hexagonal and cubic respectively. Water and ethylene glycol are adsorbed on the anodic cerium oxide film. When the anodic cerium oxide films were heat treated at 400 and 500 ℃ respectively, Ce(OH)3, Ce2O3 and Ce in the anodic cerium oxide film may be converted to CeO2, and the heat treated films are composed of CeF3 and CeO2 respectively. When the anodic cerium oxide film was heat treated at 800 ℃, Ce(OH)3, CeF3, Ce2O3 and Ce in the anodic cerium oxide film are converted to CeO2, and the heat treated film is composed of CeO2. The heat treated anodic cerium oxide film of heat treatment at 800 ℃ is CeO2 film. The CeO2 film is porous film, and the hole is a straight hole. The porous CeO2 film has strong absorption in the range of 1 600~4 000 cm-1. The coefficients of thermal expansion of the porous CeO2 film are almost constant in the range of 170~900 ℃. The porous CeO2 film has good thermal stability.
Introduction
Solid oxide fuel cell (SOFC) is an electrochemical device that converts chemical energy of fuel to electric power. SOFC is considered to be a main substitute for fossil energy resources because of its advantages such as high energy density, high efficiency conversion and low noise[1, 2, 3, 4, 5, 6]. The traditional SOFC use yttrium stabilized zirconia (YSZ) as the electrolyte[7], and the operating temperature is usually around 1 000 ℃. High operating temperature introduces a lot of problems, such as the high cost of materials, accelerating the phase reaction between the battery components, affecting the life of the battery, etc. Lowering the operating temperature is the key to the application of SOFC in practice. Replacement of YSZ with CeO2 can greatly reduce operating temperature[6, 8, 9]. Lowering the thickness of electrolyte can also reduce the operating temperature. The electrolyte film was usually supported on the electrode, especially on the anode. The preparation method of the electrolyte films are screen printing method[10], tape casting method[11], common pressure method[12], spin coating method[13], dip coating method[14], sol-gel method, plasma spray method[15], spray decomposition method, electrochemical vapor deposition method, magnetron sputtering method, and laser pulse deposition method[16], respectively. Although these preparation technologies of the films have been greatly developed in preparation and application process of the electrolyte, but there are still some problems, such as defects by calcinations, controllability of uniformity of film thickness, the holes of the films being tortuous holes and high cost. If the porous cerium dioxide films will be prepared with anodization by cerium foils as raw materials, we may solve the above problems.
In this paper, the porous CeO2 films were prepared with anodization and heat treating method by cerium foils as raw materials. The anodic cerium oxide films were heat treated at 400, 500 and 800 ℃ respectively. The crystal structures, compositions, surface morphologies of the cerium dioxide films were investigated, respectively. The properties of IR characteristic absorption and thermal expansion of the porous CeO2 film were investigated respectively.
The cerium dioxide films were prepared according to [6, 17] by anodization and heat treating method. High purity cerium foils (99.99% purity, 15 mm× 15 mm× 0.3 mm) were used as raw materials to grow anodic porous layers. First, the cerium foils were degreased using ultrasonic cleaner in absolute ethyl alcohol. Second, annealed at 500 ℃ for 3 h under a nitrogen atmosphere to remove mechanical stresses and recrystallization of the cerium foils. Third, polished with diamond spray polishing agent, followed using ultrasonic cleaner in absolute ethyl alcohol. Then to carry out anodizing experiments an electrochemical reactor was designed and built. The electrolytes were 1 mol· L-1 NaF-1 mol· L-1 NH3· H2O-aqueous ethylene glycol solution, ethylene glycol∶ H2O=10∶ 1. The anodizing parameters: lead cathode, temperature 20 ℃, time 10 h, current density 1 mA· cm-2. The anodized samples were heat treated at 800 ℃ for 2 h after anodizing, and the cerium dioxide films were got.
The crystal structures of the anodic cerium oxide film and heat treated anodic cerium oxide film were characterized with a powder X-ray diffractometer (Rigaku D/max 2 200 X, Cu Kα , 40 kV, 20 mA, 2θ : 10° ~90° ) respectively.
The compositions of the anodic cerium oxide film and the heat treated anodic cerium oxide film of heat treatment at 800 ℃ were characterized by Bruker AXS Microanalysis GmbH energy-dispersive spectrometer.
The IR characteristic absorption of the anodic cerium oxide film and the heat treated anodic cerium oxide film of heat treatment at 800 ℃ were characterized with Avatar 360 FTIR infrared spectrophotometer by potassium bromide disc method respectively.
The surface morphologies of the cerium dioxide film were determined by HITACHI S3400 scanning electron microcopy.
The physical thermal expansion coefficient of the cerium dioxide film was determined by DIL402C dilatometer, with the heating rate at 5 ℃· min-1, temperature ranges from 22~900 ℃ and argon atmosphere.
Fig.1 shows the X-ray diffraction patterns of the anodic cerium oxide films at different heat treating temperature. Table 1 shows the diffraction peaks 2θ of the films. As shown in Fig.1(a) and listed in Table 1, the anodic cerium oxide film have the diffraction patterns at 16.208° , 25.450° , 28.892° , 30.396° , 33.320° , 35.075° , 40.256° , 44.373° , 47.759° , 50.349° , 51.670° , 53.424° , 56.580° , 59.641° , 69.534° , 76.786° , 79.026° , 81.883° , 84.240° , 88.384° , respectively, the diffraction peaks correspond to hexagonal Ce(OH)3(100), CeF3(110), cubic CeO2(111), hexagonal Ce2O3(011), cubic CeO2(200), Ce(200), hexagonal Ce(OH)3(201), CeF3(300), cubic CeO2(220), hexagonal Ce(OH)3(102), CeF3(302), CeF3(221), cubic CeO2(311), CeO2(222), CeO2(400), CeO2(331), CeO2(420), hexagonal CeF3(404), CeF3(116), cubic CeO2(422) , respectively. The anodic cerium oxide film is composed of Ce(OH)3, CeF3, Ce2O3, CeO2 and Ce. Comparing Fig.1(b), Fig.1(c) to Fig.1(a), when the anodic cerium oxide film were heat treated at 400 and 500 ℃ respectively, the heat treated anodic cerium oxide films have not the diffraction patterns at 16.208° , 30.396° , 35.075° , 40.256° , 50.349° , respectively, demonstrating the transformation of Ce(OH)3, Ce2O3 and Ce in the anodic cerium oxide films into CeO2, and the heat treated anodic cerium oxide films are composed of CeF3 and CeO2 respectively. According to Fig.1d and listed in Table 1, when the anodic cerium oxide film were heat treated at 800 ℃, the heat treated anodic cerium oxide film have the diffraction patterns at 28.558° , 33.103° , 47.508° , 56.349° , 59.106° , 69.417° , 76.720° , 79.059° and 88.434° respectively, the diffraction peaks correspond to cubic CeO2(111), CeO2(200), CeO2(220), CeO2(311), CeO2(222), CeO2(400), CeO2(331), CeO2(420) and CeO2(422) respectively, demonstrating the transformation of Ce(OH)3, CeF3, Ce2O3 and Ce in the anodic cerium oxide films into CeO2, and the heat treated anodic cerium oxide film is the cerium dioxide film.
| Table 1 Diffraction peaks 2θ of films |
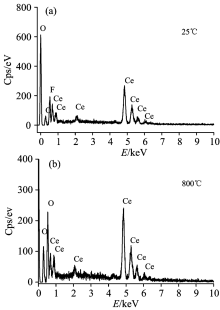
The EDAX spectra of the anodic cerium oxide film and the heat treated anodic cerium oxide film of heat treatment at 800 ℃ are shown in Fig.2. As shown in Fig.2(a), the contents of Ce(at%), O(at%) and F(at%) are 31.41%, 46.92% and 21.66%, respectively, and the anodic cerium oxide film is composed of cerium, cerium oxide and cerium fluoride. As shown in Fig.2(b), when the anodic cerium oxide film was heat treated at 800 ℃, the contents of Ce(at%) and O(at%) are 32.40% and 67.60% respectively, and Ce(at%)∶ O(at%)=1∶ 2, the heat treated anodic cerium oxide film is CeO2 film. The result is consistent with the XRD analysis result.
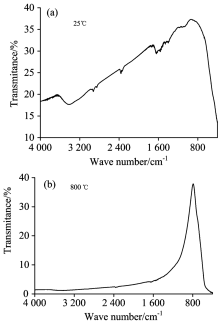
The FTIR spectra of the anodic cerium oxide film and the heat treated anodic cerium oxide film of heat treatment at 800 ℃ are shown in Fig.3. As shown in Fig.3(a), there are ν O— H vibration peak at 3 418.35 cm-1,
| Table 2 The locations of infrared absorption spectra of the peaks (cm-1) |
The surface morphologies of the cerium dioxide film are shown in Fig.4. It is seen in Fig.4 that, the structure of the cerium dioxide film is the porous, and the holes are straight holes.
Fig.5 shows the physical thermal expansion curves of the porous CeO2 film. According to Fig.5, the coefficients of thermal expansion of the porous CeO2 film are almost continuous in the range of 170~900 ℃. The porous CeO2 film has good thermal stability.
The anodic cerium oxide film is composed of Ce(OH)3, CeF3, CeO2, Ce2O3 and Ce, and the crystal structures of Ce(OH)3, CeF3 , Ce2O3 and CeO2, Ce are hexagonal and cubic respectively. Water and ethylene glycol are adsorbed on the anodic cerium oxide film. When the anodic cerium oxide films were heat treated at 400 and 500 ℃ respectively, Ce(OH)3, Ce2O3 and Ce in the anodic cerium oxide film may be converted to CeO2, and the heat treated films are composed of CeF3 and CeO2 respectively. When the anodic cerium oxide film was heat treated at 800 ℃, Ce(OH)3, CeF3, Ce2O3 and Ce in the anodic cerium oxide film are converted to CeO2, and the heat treated film is composed of CeO2. The heat treated anodic cerium oxide film of heat treatment at 800 ℃ is CeO2 film. The CeO2 film is porous film, and the hole is a straight hole. The porous CeO2 film has strong absorption in the range of 1 600~4 000 cm-1. The coefficients of thermal expansion of the porous CeO2 film are almost constant in the range of 170~900 ℃. The porous CeO2 film has good thermal stability.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|