研究了一种含咔唑取代基喹啉铝(Alq3)衍生物的发光性能。 首先, 研究了溶剂对其发光性能的影响。 研究发现, 在不同溶剂体系中, Alq3衍生物在发光过程中均存在不同程度的能量传递; 但是, 溶剂影响Alq3衍生物的空间结构和电子结构, 进而影响能量传递效率, 导致激发光谱、 发射光谱、 荧光效率和荧光寿命均不相同。 其中, 甲苯起到能量传递桥梁的作用; 而DMF在一定程度上阻碍能量传递。 与DMF结构类似的DMA作为电子给体加入到Alq3衍生物溶液中, 也起到了类似作用。 另外, 研究不同金属离子对Alq3衍生物发光性能的影响发现, Fe3+和Cu2+可引起Alq3衍生物的荧光猝灭, 表明该Alq3衍生物可以作为多功能荧光传感材料。
This work presents a study on the luminescence properties of a carbazole-substituted aluminum quinolone (Alq3) derivative. Firstly, the effect of solvents on the luminescence properties of the Alq3 derivative was studied. The results indicate that energy transfer can occur from carbazole to Alq3 for the Alq3 derivative in all different solvent systems. However, solvent affects the spatial structure and electronic structure of the Alq3 derivative that have an impact on the efficiency of energy transfer, leading to different luminescence spectra, efficiency and lifetime for different solvent systems. Among them, toluene plays a role of bridge in energy transfer, but a large amount of DMF plays negative role in energy transfer. Like DMF, DMA acting as an electron donor also plays similar role. Besides, effects of metal ions on the luminescence properties of the Alq3 derivative were also studied. The results show that Fe3+ and Cu2+ can cause fluorescence quenching, and the fluorescence quench effect is also affected by acid counteranions. These results indicate that the Alq3 derivative might be exploited as a multifunctional fluorescent sensing material.
Introduction
Aluminum quinolone (Alq3) and its derivatives have attracted considerable attention because of their excellent photophysics properties and potential applications in displays and sensors[1, 2]. In recent decades, different electron donating or electron withdrawing substituents have been introduced to the 2, 5 and 7 positions of the quinoline ring to modulate the photophysics properties of Alq3[3, 4, 5, 6, 7, 8].Carbazole and its derivatives as a kind of excellent photoelectric functional groups, have also been introduced to Alq3[6, 7, 8]. In our previous work, an Alq3 derivative containing carbazole substituents has been prepared[8]. In this carbazole substituted Alq3 derivative, cabazole was attached at 5-position of quinolone ring with methylene (— CH2— ). According to literature reports, the luminescence of Alq3 is based on the π — π * transition from the electron-rich phenolring to the electron-deficiency pyridine ring[9]. Moreover, the HOMO orbital is concentrated in the phenol ring, especially in the C5 position of the ring. Therefore, carbazole attached at the 5-position of phenol ring has a great influence on the luminescence of Alq3-center, which may be influenced by external factors such as solvents and metal ions.
Solvent-dependent luminescence behavior (solvate effect) of metal complexes have attracted considerable attention because of their potential applications in sensors and display[10, 11, 12, 13, 14]. Recently, Tan et al. reported a series of lanthanide-organic complexes based on polyoxometalates, which represented different emission intensity due to the difference of the solvent permittivity[10]. The correlation between luminescence property of Au(Ⅰ )-SR (SR: thiolate) complexes and solvent polarity was also studied by Yang et al. The results is proposed that the luminescence of Au(Ⅰ )-SR complexes originates from the aggregation of the bilayer supramolecular structures induced by the weakly polar solvent. This aggregation strengthens the intra and intercomplex aurophilic Au(Ⅰ )…Au(Ⅰ ) interactions and subsequently enhances the luminescence intensity of the complexes[11]. Theoretical calculations shows that the highest and lowest energy absorption bands of Bu4N[(4, 4’ -bpy)Re(CO)3 (bpy-5, 5’ -diCOO)] experience a bathocromic shifts as the polartity of the solvent decreases[12]. A Cu(Ⅰ )-based complex [Cu7(CN)7(4, 4’ -bipy)] demonstrates solvent-dependent luminescent behaviors in various solvents due to the effects of solvent molecules on the frontier molecular energy levels[13]. A cuprous cyanide coordination framework bearing auxiliary PymNH2 ligand demonstrates solvent-dependent luminescent behaviors in various solvents only via metal-solvent coordination interaction[14]. Thus it can be seen that the influence of solvent on the luminescence of these metal complexes is perplexing, which is worth further studying.
Herein, the correlation between luminescence properties of the carbazole-substituted Alq3 derivative and solvent was investigated carefully. The dependence of luminescence intensity on the mixed solvent with various DMF/acetone ratios was also explored. In addition, effects of electron donors (DMA) and metal ions on the luminescence properties of the carbazole-substituted Alq3 derivative were also investigated in detail, which lay the foundation for their application in the field of fluorescence sensing.
Carbazole-substituted Alq3 derivative (Fig.1) was prepared according to our previous work[8]. N, N-dimethylacetamide (DMA), sodium chloride, potassium chloride, calcium chloride, barium chloride, aluminum chloride, ferric chloride, copper chloride, cobalt chloride were purchased from domestic chemical reagent companies, and untreated before use. Acetone, chloroform, toluene, acetonitrile, N, N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were also purchased from domestic chemical reagent company, and treated according to standard methods prior to use. Copper perchlorate was prepared according to standard methods.
UV-Vis absorption spectra were determined on a Shimadz spectrophotometer. Excitation and emission spectra were measured by a Hitachi F-4600 fluorescence spectrophotometer. Fluorescence quantum efficiencies were determined in different solvents with the concentration of 10-5 mol· L-1 by relative method using quinine sulfate as the standard. Fluorescence lifetimes were measured by the time-correlated single photo-counting method (FLS 920 steady state spectrophotometer) with excitation at 405 nm and the emission was monitored at 530 nm.
The absorption data of the Alq3 derivative in different solvents (10-5 mol· L-1) are shown in Table 1. All of the absorption spectra have three main absorption peaks around at 329, 342 and 395 nm. In addition, a characteristic peak of carbazole located at 294 nm can be find in all solvent systems except acetone. The absorption peaks around 294, 329 and 342 nm are attributable to the 1A→ 1Ba, 1A→ 1Lb transition of carbazole, respectively. The peak of 395 nm is originated from π — π * electron transition from phenol ring to pyridine ring, which is red shifted about 20 nm when compared with the non-substituted Al
| Table 1 Absorption and photophysical data of the Alq3 derivative in different solvents (10-5 mol· L-1) |
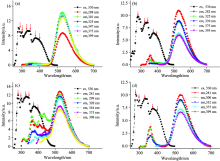
Excitation and emission spectra of the Alq3 derivative in different solvents (10-5 mol· L-1) are shown in Fig.2. The excitation spectra are similar in chloroform, acetonitrile, DMF, and DMSO, which have five main excitation peaks around 281, 300, 327, 342 and 375 nm. The first four peaks are identified as the characteristic peaks of carbazole, and the last one is attribute to the characteristic excitation of Alq3. In toluene, the excitation peak located at 281 nm red shift to 287 nm and accompanied by intensity decrease. In acetone, the excitation band becomes narrow (320~470 nm), and only two excitation peaks can be find around 342 and 375 nm. Together with the absorption data, one can speculate that the presence of acetone cause slight change of the coordination environment as the previous report[10]. However, it can be seen that the Alq3 derivative in all solvents show the characteristic excitation peaks of carbazole, indicating that energy transfer occurs from carbazole to Alq3-center. Also, energy transfer can be affected by solvent to some extent, which lead to different excitation spectra in different solvent.
The emission spectra of the Alq3 derivative in different solvents (10-5 mol· L-1) are shown in Fig.2(b). All of these spectra exhibit intense emission of Alq3 around 530 nm and weak peaks of carbazole, which also evidences the occurrence of energy transfer from carbazole to Alq3-center. With the increase of the polarity of the solvent, the characteristic peak of Alq3 has a slight red-shift as a result of solvent effect[10]. In toluene, only the characteristic peak of Alq3 can be find in Fig.2(b), which is due to the efficient energy transfer from carbazole to Alq3-center. However, the characteristic peak of carbazole can be find around 433 nm in DMF and chloroform. This may be due to the fact that the polar solvent affects the coordination structure of the Alq3 derivative to some extent, as described above. Besides, the effect of solvation also affects the spatial structure and electronic structure of the Alq3 derivatives, which finally affects the efficiency of energy transfer from carbazole to Alq3.
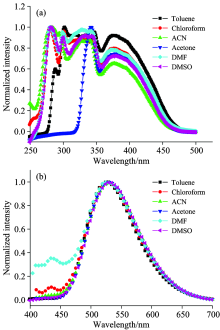 | Fig.2 (a) Excitation (λ em=530 nm) and (b) emission (λ ex=375 nm) spectra of the Alq3 derivatives in digferent solvents |
In order to further study the effect of solvents on the energy transfer from carbazole to Alq3, the emission spectra were determined under different excitation light, as shown in Fig.3. In toluene, whether it is excited with the characteristic excitation light of carbzole or that of Alq3, the Alq3 derivative only displays the characteristic emission of Alq3, and the characteristics emission of carbazole disappear completely. The results further indicate that the effect of non-polar solvent on the spatial structure and electronic structure of the Alq3 derivative can be neglected, and the energy transfer from carbazole to Alq3 cannot be affected obviously by nonpolar solvents. Instead, the non-polar toluene may induce the Alq3 derivative to form aggregation, which leading to the enhanced luminescent intensity[11]. In polar solvents, the emission spectra were determined through exciting with different excitation light, as shown in Fig.3(b-d). The characteristic emission of both Alq3 and carbazole can be seen in these spectra, but the emission intensity of carbazole is relatively weak. The excitation wavelength is shorter, and the emission peak of carbazole is stronger. One can also find that there is a single peak of carbazole around 365 nm in ACN and DMSO, but there are multiple peaks in DMF. The results reveal that the effect of DMF on the luminescence of the Alq3 derivative is intricate and worth farther study.
The luminescence data of the Alq3 derivative in different solvents (10-5 mol· L-1) are shown in Table 1. In toluene, the fluorescence quantum efficiency (φ f=3.37%) is the highest, which is close to that of Alq3 (φ f=4%). These decay curves of the Alq3 derivative in different solvents (10-5 mol· L-1) can be fitted by double-exponential function. The values of the lifetime (τ 1 and τ 2) and the corresponding fraction (α 1 and α 2) are listed in Table 1. According to the values of the lifetime and the corresponding fractions, the average PL lifetimes of the Alq3 derivative in different solvents were calculated according to the equation: < τ > =(a1
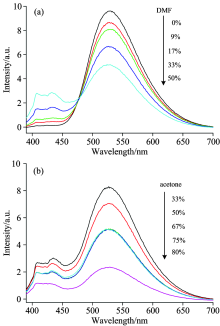
DMF was gradually dripped into the acetone solution of the Alq3 derivative (10-5 mol· L-1), and the emission spectrum were measured as shown in Fig.4(a). Exciting with 375 nm, the Alq3 derivative only emit the characteristic emission of Alq3 in acetone. With the addition of DMF, the characteristics emission of Alq3 decreased gradually and the characteristic peak of carbazole started to appear. Simultaneously, the emission intensity of carbazole gradually enhanced with the addition of DMF. The results reveal that the solvation effect of acetone on the Alq3 derivative is weaker than that of DMF. The interaction of the Alq3 derivative and acetone may be replaced by that of Alq3 derivative and DMF. Alternatively, hydrogen bonds may be formed between the hydroxyl group of quinolone and DMF. Both of the two cases weakened the coordination of aluminum ions. Moreover, the interaction between carbazole and DMF may be formed to hinder the energy transfer from carbazole to Alq3, eventually leading to enhance the emission intensity of carbazole.
The emission spectrum changes of the Alq3 derivative in DMF (10-5 mol· L-1) with the addition of acetone were recorded as shown in Fig.4(b). Exciting with 375 nm, the characteristic emission of both Alq3 and carbazole can be find in the emission spectra of the Alq3 derivative in DMF. With the addition of acetone, the characteristics emission of both Alq3 and carbaozle decreased gradually, but the former is falling faster. The phenomenon can be explained that strong interaction have been formed between the Alq3 derivative and DMF, adding acetone only play a dilution effect that have little impact on the spectrum.
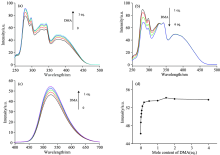
In order to further study the effect of DMF on the luminescence properties of the Alq3 derivative, N, N-dimethylacetamide (DMA), similar to DMF as electron donor, were added to the chloroform solution of the Alq3 derivative. With addition of DMA, both excitation and emission spectra of the Alq3 derivative slightly enhanced as shown in Fig.5(a) and (c). As DMA dropping continuously, the excitation peak of carbazole decreased gradually, while the excitation peak of Alq3 remain unchanged, as shown in Fig.5(b). In Fig.5(d), the emission intensity of Alq3 increases at first, and then levels off with the increase of DMA. These phenomena indicates that the DMA as electron donor is beneficial to the luminescence of the Alq3 derivative when the amount of DMA addition is less than one time, similar to the previous report[6]. With increasing of the amount of DMA, energy transfer from carbazole to Alq3 have been hindered in a certain extent. As solvent, DMF dosage is relatively large, which leads to relatively large negative effect for the energy transfer from carbazole to Alq3, and then decreasing the luminescence efficiency of Alq3.
Several metal chloride (NaCl, KCl, CaCl2, BaCl2, AlCl3, MgCl2, FeCl3, CuCl2, CoCl3) were added to the acetone solution of the Alq3 derivative (2× 10-5 mol· L-1), and the emission intensity of the Alq3 derivative are presented in Fig.6. One can find that both Fe3+ and Cu2+ exhibit apparent quenching effect on the luminescence of the Alq3 derivative. The result shows that the Alq3 derivative can be used as fluorescence probe for metal ions.
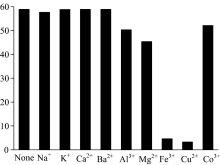 | Fig.6 Emission intensity of the Alq3 derivative in acetone (2× 10-5 mol· L-1) with the addition of 15 eq. metal chloride (λ ex=375 nm) |
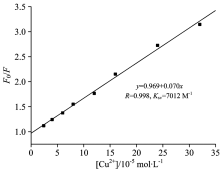
Excitation and emission spectra of the Alq3 derivative in acetone (2× 10-5 mol· L-1) with the addition of CuCl2 are shown in Fig.7. With the addition of CuCl2, both excitation and emission peaks were not shifted, but the intensity of all peaks decreased gradually (fluorescence quenching effect). The emission intensity of the Alq3 derivative without CuCl2 was designed as F0, and that of the Alq3 derivative with different amounts of CuCl2 was designed as F. The change curve of the ratio of F0/F to the amounts of CuCl2 was described in Fig.8. One can find that the intensity ratio is linear with the amount of CuCl2.
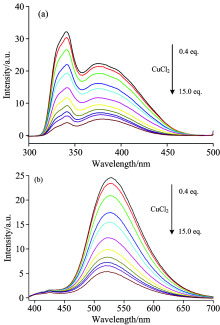 | Fig.7 (a) Excitation (λ em=530 nm) and (b) emission (λ ex=375 nm) spectra of the Alq3 derivative in acetone (5× 10-5 mol· L-1) with addition of CuCl2 |
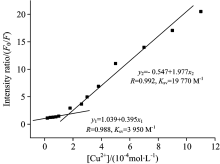
Excitation and emission spectra of the Alq3 derivative in acetonitrile (5× 10-5 mol· L-1) with addition of Cu(ClO4)2 are shown in Fig.9. With the addition of Cu(ClO4)2, the excitation peaks have hardly shifted, but the emission peaks have a blue-shift. It shows that the electronic structure of the luminescence center has been changed, which may be due to the oxidation of Cu(ClO4)2 in acetonitrile[15]. In addition, it is obvious that the intensity of both excitation and emission peaks gradually decreased with the increase of Cu(ClO4)2, which is the fluorescence quenching effect. The emission intensity of the Alq3 derivative without Cu(ClO4)2 was designed as F0, and the emission intensity of the Alq3 derivative with different amounts of Cu(ClO4)2 was designed as F. The change curve of the ratio of F0/F to the amounts of Cu(ClO4)2 was also described as shown in Fig.10. It can be found that this curve is divided into two parts by the dividing point of [Cu(ClO4)2]=1.5× 10-4 mol· L-1 (that is 3 times of the Alq3 derivative), and each sections show a linear relationship respectively. The different phenomenon also demonstrate that carbazole may be oxidized in acetonitrile with Cu(ClO4)2.
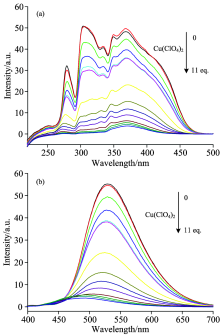 | Fig.9 (a) Excitation (λ em=530 nm) and (b) emission (λ ex=375 nm) spectra of the Alq3 derivative in acetonitrile (5× 10-5 mol· L-1) with addition of Cu(ClO4)2 |
Luminescent properties of carbazole-substituted Alq3 derivative were studied in detail. Upon irradiation with UV light, the Alq3 derivative exhibit intense emission of Alq3 around 530 nm and weak peaks of carbazole, which evidences the occurrence of energy transfer from carbazole to Alq3-center. Toluene plays the role of bridge for energy transfer, and DMF plays multiple roles in the luminescence of the Alq3 derivative. A small amount of DMF or DMA as an electron donor, plays a role in the promotion of luminescence, but a large amount of DMF or DMA reduce the energy transfer efficiency and luminescence efficiency due to solvation effect. Besides, Fe3+ and Cu2+ can cause the fluorescence quenching, which also affected by acid counteranions. These results indicate that the Alq3 derivative might be exploited as a multifunctional fluorescent sensing material for the detection of organic solvents and metal ions.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|