Hybrid nanomaterials have attracted considerable interest in environmental science, analytical chemistry and atomic spectroscopy. In the present study, a column solid phase extractioo procedure was developed for the separation and preconcentration of indium in various matrixes by using hybrid nanomaterial B2O3/ZrO2 (HNMBZ). Various experimental and analytical parameters such as sample solution pH, sample solution volume, flow rate of sample solution and eluent, volume and concentration of eluent aod amount of HNMBZ, effect of common matrix ions and capacity of sorbent were investigated. The adsorbed metal ions on HNMBZ were eluted with 6 mL of 1 mol·L-1 HNO3 solutions and their concentrations were determined by high-resolution continuum source flame atomic absorption spectrometry (HR-CS FAAS). Under the optimized conditions, detection limits for indium for 3 pixels and 5 pixels were found as 0.20 and 0.16 μg·L-1, respectively. The accuracy of the procedure was checked by spiked water samples. The developed procedure was successfully applied to real samples for the separation and determination of indium.
Introduction
Indium is an important element in the semiconductor industry, in the nuclear studies and in the production of high purity materials. It is a rare and valuable metal that is used in a variety of industrial applications, such as liquid crystal displays (LCDs), semiconductors, low-temperature solders, infrared photodetectors, and solar cells[1, 2, 3]. Indium is a crystalline, very soft, ductile, and malleable metal that retains its high plastic properties at cryogenic temperatures. In general, increases the strength, corrosion resistance, and hardness of an alloy system, when indium is added. Indium is also used in electrical contacts, liquid crystal displays, low-pressure sodium lamps, alkaline dry batteries, and semiconductors[4]. However, there are no discrete reserves of indium, and its global distribution is very sparse. Since the use of this metal is likely to increase in the near future, it is becoming more important the development of analytical methods to determine and recover indium in various matrixes[3].
Indium is widely spread in nature, generally is it in very low concentrations. The direct determination of extremely low concentration of indium is not limited only with low sensitivity, but it is also limited with matrix interference. For this reason, the preliminary separation and preconcentration of trace indium element from matrix is often required. Separation and preconcentration procedures have very important role for sample preparation processes in analytical methods[5, 6].
In the literature, a number of separation and preconcentration procedures, such as cloud point extraction (CPE), solid-phase extraction (SPE), co-precipitation, dispersive liquid-liquid micro-extraction have been used for trace metals[7, 8, 9, 10]. Among these separation-preconcentration techniques, SPE has achieved an increasing application, because of its simple procedure, higher preconcentration factor, rapid phase separation and combination with different detection techniques[11]. Many metal species can be preconcentrated and determined, when SPE is used in batch or column techniques[12, 13, 14].
In SPE process for trace elements needs solid sorbent materials, some of which are Amberlite and Duolite XAD resins, activated carbon, cellulose, modified silica gel, polymeric resin, biomass and nanomaterials[15].
Due to their advantages of large surface area, chemical stability, durability, corrosion resistance, and cost effectiveness, nanomaterials have gained the greatest interest over other materials as the solid phase for SPE to improve the sensitivity of atomic spectrometry and/or minimize the interferences from complex matrices[16].
In this study, hybrid nanomaterial B2O3/ZrO2 (HNMBZ) has been used as sorbent, which is synthesized by our research group[17] for developing a new solid phase extraction method for the trace indium metal in various matrixes. Some analytical parameters were investigated and optimized. The developed procedure was successfully applied to water samples.
The analysis was performed by ContrAA 300 a High Resolution-Continuum Source Flame Atomic Absorption Spectrometer (HR-CS AAS) (Analytik Jena AG, Jena, Germany) equipped with a 50 mm burner head. Air-acetylene flame was used as an atomizer. All absorption lines for an element in the spectral range of 185~900 nm can be analytically evaluated by using a Xe short-arc lamp as a continuum lamp source. Detector technology of HR-CS FAAS is based on a CCD chip, which is used for the first time in AAS. The pixels are illuminated and read out simultaneously and act as independent detectors. 200 pixels of the chip are used for the analytical function. All corrections necessary in AAS are performed by reference pixels. In HR-CS AAS with a CCD detector, the center of the absorption line is focused on the center evaluation pixel. The evaluation width acts as a new variable here and offers optimizing options. With the help of the absorbance, measured at several pixels, the sensitivity, reproducibility and the linear working range can be influenced as a function of the absorption line width.
The spectral background of the sample in the HR-CS FAAS is always corrected directly on the analysis line simultaneously and independently. All measurements were carried out under optimum conditions in three replicates using an enabling the computer controlled aspiration of blanks, analytical solutions and samples. pH of the aqueous solution was measured by an Orion Star (Thermo Fisher, USA) model pH meter. The operating conditions of HR-CS FAAS for indium metal by are given in Table 1.
| Table 1 The operating conditions for HR-CS AAS for indium determination |
All reagents were of analytical grade unless otherwise stated. All solutions were prepared using ultra pure water (specific resistance 18 MΩ cm) from a Milli-Q purification system (Millipore Corporation, Massachusetts, USA). Standard solutions of analytes were prepared from their 1 000 mg· L-1 stock solutions (Merck).
Acetic acid/acetate buffers (0.1 mol· L-1) were prepared by adding an appropriate amount of acetic acid to ammonium acetate solutions to result in solutions of pH 4~6. For pH 7, sodium monohydrogen phosphate-potassium dihydrogen phosphate (0.1 mol· L-1) buffer solution was used. Ammonia/ammonium chloride buffer solutions (0.1 mol· L-1) were prepared by adding an appropriate amount of ammonia to ammonium chloride solutions to result in solutions of pH 8~10. All glassware was cleaned using ultra pure water, kept in nitric acid for 24 h, and washed again with ultra pure water.
HNMBZ was synthesized by modifying the procedure given in the literature[17]. 10 g of H3BO3 and 5 g of Zr(OCl)4 were weighed into a beaker containing about 50 mL of ethanol. Then, 1.5 mL of Triton X-114 was added to the mixture as surfactant and stirred for 1 h. The mixture was sonicated for about 30 min in ultrasonic bath. pH of the mixture was adjusted to 6 and then sonication was applied again for 15 min. The mixture was left for 12 h at room temperature and then dried in an oven at 70 ℃ for about 1 h. The obtained solid material was then transferred into the muffle furnace and heated at 850 ℃ for about 2 h. Then, the material was ground in a Spex type ball mill to obtain powder product.
A glass column, which was 10.0 cm in length and 0.8 cm in internal diameter and had a 250 mL reservior on top of the column and a stopcock at the bottom of the column, was used for separation/preconcentration of trace indium element. Column system was prepared by placing a small portion of cleaned glass wool as a plug at one end of the column holding a certain amount (0.2~0.5 g) of HNMBZ. Column system was rinsed with water and 2 mol· L-1 HCl solutions, respectively[18, 19].
The column SPE procedure was tested with model solutions prior to the determination of trace indium element in real samples as off-line. For a model solution, 5 mL of 2.0 mg· L-1 indium (Ⅲ ) solution and 4 mL of appropriate buffer solutions were added in a 50 mL volumetric flask to obtain the desired pH between 3 and 9. Then, final volume was completed to 50 mL with deionized water. The sorbent bed was first washed with distilled water, and then pre-conditioned by passing the blank solutions of working pH through column, and then the model solution was passed through the column at a flow rate of 4 mL· min-1 by means of a peristaltic pump. Afterwards, the column was rinsed with 10 mL of water, and the adsorbed indium ions on HNMBZ were eluted with 6 mL of 1 mol· L-1 HNO3 solutions. The eluent was collected, and then the indium concentrations were determined by using HR-CS FAAS. The recovery of the indium was calculated from the ratio of the concentration found by HR-CS FAAS and the concentration calculated theoretically.
A tap water sample was collected from a tap in one of the laboratories at Ahi Evran University. Thermal water was collected from Kı zı lcahamam Thermal Water in Ankara-Turkey. Most frequently consumed mineral and drinking water were bought directly from local supermarkets. The water samples were filtered through a cellulose membrane filter (Millipore) of pore size 0.45 μ m. The pH of the samples was adjusted to 7.0 using sodium monohydrogen phosphate-potassium dihydrogen phosphate buffer solution/ To obtain spiked samples, known amounts of indium(Ⅲ ) were added to the water sample. Then, the preconcentration procedure was applied to the unspiked and spiked samples.
To obtain quantitative recovery of indium ions on HNMBZ, the procedure was optimized for various analytical parameters, such as the pH of sample solution, the type and concentration of eluent, the volume of sample solution, and the flow rate of sample and eluent solution. The analytical parameters, such as linear dynamic range, limit of detection (LOD), limit of quantification (LOQ), precision and accuracy have been determined under optimal conditions. The effect of foreign ions has also been investigated.
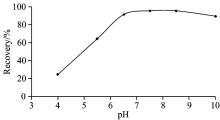
It is known, that hydrogen ion concentration plays an important role in the adsorption of metal ions on the sorbent, because it significantly affects the binding of metal[20]. The pH!values of the “ model solutions” described above were adjusted to appropriate pH in the range of 3.0~9.0, separately. The obtained results were given in Fig.1. The quantitative recovery (above 95%) of indium ions were obtained at pH 7.0~8.5. The recoveries of In3+ were decreased when the solutions pH is over 8.5 and less than 7.0 (Fig.1). Hence, pH 7.0 was selected as an optimum pH for solid phase extraction of the indium ions for further experiments.
At lower pH values (pH< 7.0), owing to the competition between protons and the metal ions the recoveries decrease. At the higher pH values above pH 8.5, formation of precipitates and/or anionic hydroxide complexes may occur.
Acid solutions are widely used for the elution of metal ions from a sorbent due to the protonation at a chelating site of the sorbent. Among acids, nitric acid replaces the metal ions from binding sites, and hence is commonly used. In order to choose the most effective eluent for desorbing of indium ions from the HNMBZ, different concentration and different volumes HCl and HNO3 solutions were tested. Quantitative recovery (> 95%) for indium has been obtained by using 6 mL of 1 mol· L-1 HNO3 solution as an eluent (Table 2).
| Table 2 The effect of eluent type/concentration/volume of eluent on recovery of indium |
aMean value and standard deviation of three replicate analyses
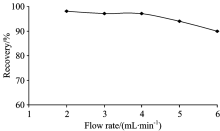
The flow rate of the sample solution also effects on the recovery of indium ions. Therefore, the effect of flow rate was investigated under the optimum conditions (pH 7; eluent 6 mL of 1 mol· L-1 HNO3). Samples were then passed through the column at a flow rate that varied from 2 to 6 mL· min-1 adjusted by peristaltic pump. As shown in Fig.2, the optimum value for the flow rate of the sample solution was found as up to 4 mL· min-1. Above 4 mL· min-1, the recovery values of indium decreased gradually. Therefore, to decrease the duration time without decreasing recovery values, a rate of 4 mL· min-1 was chosen as the optimum flow rate for subsequent experiments.
The influences of the sample volume on the recovery of indium were also investigated. For the preconcentration purposes, to achieve the higher preconcentration factor, the eluent volume should be as small as possible, and the volume of sample solution should be as high as possible[11].
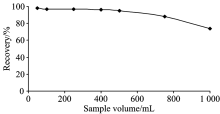
In order to obtain the maximum applicable sample solution (or analyte concentration), model solutions including the same amount of indium ions with different volumes were used. For this purpose, aqueous solutions containing 5 μ g indium (Ⅲ ) ions were preconcentrated from the sample volumes ranged from 100 to 1 000 mL (corresponding the concentration range of 50 to 5 μ g· L-1). The results are given in Fig.3. The recoveries of indium was quantitative (> 95%) for sample volumes up to 500 mL. Preconcentration factor (PF) was calculated by the following equation
where VS is the volume of analyte solution to be preconcentrated (here 500 mL) and VE is the volume of eluate (6 mL). Maximum preconcentration factor for indium (Ⅲ ) was found as 83.3.
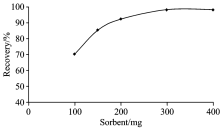
The influence of amount of HNMBZ on the recovery of indium (Ⅲ ) were also investigated by using sorbent in the range of 100~400 mg. 50 mL of model solution including 5 μ g of indium was passed through the column at optimum experimental conditions (pH 7; flow rate 4 mL· min-1; eluent, 6 mL of 1 mol· L-1 HNO3). The obtained results showed that the recovery of the indium increase up to 300 mg of HNMBZ and remain about constant above this value (Fig.4). Therefore, minimum sorbent amount (300 mg) resulted maximum recovery was selected for further studies.
The adsorption capacity is an important factor in the evaluation of the property of a sorbent, because it determines how much sorbent is required for obtaining quantitative recovery of the analyte ions from a given solution[21, 22, 23]. Adsorption capacity of the sorbent for indium is calculated as follow:
Where, Q is the adsorption capacity in mg· g-1, Ci is the initial concentration of indium in mg· L-1, Cf is the final concentration of indium after adsorption process in mg· L-1, V is the sample volume in L and m is the amount of sorbent in g.
To determine adsorption capacity, 0.1 g of HNMBZ was added into a beaker containing 50 mL of aqueous solution including 50 mg of indium (Ⅲ ) ion and buffer solution at an optimum pH and stirred for 60 min with a magnetic stirrer. Then, indium in supernatant solution was determined by using HR-CS FAAS after needed dilution process. The adsorption capacity of sorbent for indium (Ⅲ ) was found as 286 mg· g-1.
Matrix effects are important problem in the determination of heavy metals by atomic spectroscopic techniques in real samples. To investigate the possibility of selective recovery of indium on HNMBZ in the presence of some metal ions, the presented procedure has been performed with model solutions containing these ions. 50 mL of model solutions containing 5 μ g of indium (Ⅲ ) ions was used for this purpose. The maximum concentrations of the various metal ions as their nitrate or chloride salts which are tolerable within the 5% relative error were determined by adding them to a solution containing of indium ions and by applying the proposed procedure. The results indicated that the foreign ions tested having concentrations given in Table 3 did not interfere significantly with recovery of indium ion.
| Table 3 Effects of the some foreign ions on the recovery of indium |
Under the optimum experimental conditions, linear dynamic range, correlation coefficient, LOD, LOQ, precision and accuracy were examined. By using direct aspiration in HR-CS FAAS without applying the preconcentration system the linear dynamic range for Indium determination for evaluation pixels as 3 pixels and 5 pixels were 0.05 to 5.00 mg· L-1 (R2=0.997 8) and 0.04 to 5.00 mg· L-1 (R2=0.999 8), respectively.
LOD of this procedure was determined as the concentration corresponding to three and ten times of the standard deviation (σ ) of blank measurements (N=20). The obtained LOD values without applying the preconcentration procedure was divided to the preconcentration factor in order to obtain the LOD values of the proposed method[24, 25]. LOD [3σ /(m× PF)] for 3 pixels and 5 pixels were found as 0.20 and 0.16 μ g· L-1, respectively. Where, m is slope of calibration curve obtained without preconcentration procedure and PF is preconcentration factor for the proposed procedure.
The precision of this procedure was examined by seven replicate measurements of 50 mL of sample solutions including 100 μ g· L-1 indium (Ⅲ ). The mean indium recoveries for 3 pixels and 5 pixels were obtained as 98.4% and 99.1%, respectively with the relative standard deviations (RSD) of 1.8% and 1.2%, respectively. The accuracy of the proposed method was checked by analyzing spiked samples. Results are given in Section 3.10.
The stability and reusability of the HNMBZ were evaluated by determining the recoveries of the analytes by applying adsorption-elution cycles. One adsorption-elution cycle was performed as follow: the passage of 50 mL of the model solution, 6 mL of eluent solution and 50 mL of ultra pure water through the column loaded with 300 mg of sorbent, respectively. The sorbent was always stored in water when it was not in use. It was observed that the sorbent was stable up to 50 cycles without major loss in its quantities and metal recovery properties.
The improved method was applied for the determination of indium in water samples. The validity of the proposed method was further proven by analyzing spiked analyte ions samples. The results obtained are given in Table 4. A good agreement obtained between added and found value of indium indicates that the accuracy of the method is satisfactory. Percent errors below 5% are usually acceptable for trace metal analysis for analytical purposes.
| Table 4 Mass concentrations of indium in drinking, tap and mineral waters and their recoveries from spiked water samples |
Some comparative data about Indium preconcentration by other sorbents and ligands are summarized in Table 5. Analytical characteristics obtained for this study are comparable to many procedures given in this table. The LODs of present procedure are better than some of the other reported preconcentration methods[27, 29, 30, 31]. Preconcentration factor is relatively higher than similar procedure reported in literature[26, 28, 30, 31, 32] and the RSD (%) of this work is comparable to given procedure in Table 5.
| Table 5 Comparison of the proposed method for preconcentration of Indium ions in aqueous solution with other methods described in the literature |
A solid phase extraction procedure was suitable for the preconcentration and direct determination of indium in water samples on nano B2O3/ZrO2 by high resolution continuum source atomic absorption spectrometry. This method was a high selective adsorbability for indium at certain pH, high precision and concentration factor and low detection limit. Moreover, the effects of coexisting interfering ions in the determination of In could be eliminated by the selective adsorption, elution and sample preparation. So this method can be applied to determine indium in water samples.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|