采用紫外-可见光谱法(UV-Vis)研究Fc(COOH)2 ( λmax=255 nm)与BSA( λmax=277.5) 的相互作用。 实验结果表明: Fc(COOH)2在10~190 μmol·L-1范围内吸光度与浓度呈良好的线性关系( r=0.998 4), BSA在100~1 900 mg·L-1范围内, 吸光度与浓度呈良好的线性关系( r=0.999 2), BSA与Fc(COOH)2反应后, 最大吸收波长移至275 nm。 当固定Fc(COOH)2或BSA的浓度时, Fc(COOH)2或BSA的吸光度随着BSA或Fc(COOH)2浓度的增加而增大, 说明Fc(COOH)2与BSA存在分子间的相互作用, 主要是由于Fc(COOH)2和 BSA能形成氢键, 分子链增长, 吸收的能量增加, 导致吸光度增大。 同时考察Fc(COOH)2和 BSA的吸光度随时间的变化, 70 μmol·L-1的Fc(COOH)2与1 900 mg·L-1的BSA反应0.1, 24和96 h后, 在 λmax=275 nm处的吸光度由1.062分别变为1.045和0.986; 当700 mg·L-1的BSA与190 μmol·L-1的Fc(COOH)2反应0.1, 24和96 h后, 在 λmax=275 nm处的吸光度由0.813分别变为0.794和0.750。
The interactions between Fc(COOH)2 ( λmax=255 nm) and BSA ( λmax=277.5 nm) were studied by UV-Vis spectroscopy. The results showed that, there was a good linear relationship between absorbance and concentration for Fc(COOH)2 in range of 10~190 μmol·L-1 and for BSA in range of 100~1 900 mg·L-1, respectively. After the reaction of Fc(COOH)2 and BSA , λmax shifted to 275 nm to show the existing reaction. When the concentration of Fc(COOH)2 or BSA was fixed, the absorbance of Fc(COOH)2 or BSA increased with the increase of BSA or Fc(COOH)2 concentration, which showed the hydrogen bonding form between Fc(COOH)2 and BSA and the growth of molecular chain, and the increase of absorbance was attributed to the fact that bigger molecule absorbs more energy and release. Meanwhile, the stability of new formed molecule bond was affected by reaction time, after reacted for 0.1 to 24 and 96 h, the absorbance at 275 nm changed from 1.062 to 1.045 and 0.986 for Fc(COOH)2 (70 μmol·L-1) and BSA (1 900 mg·L-1), and the absorbance changed from 0.813 to 0.794 and 0.750 for Fc(COOH)2 (190 μmol·L-1) and BSA (700 mg·L-1), respectively.
Bovine serum albumin (BSA) is often used as a protein concentration standard in lab experiments which is derived from cows[1], and it is a byproduct of the cattle industry, since large quantities of BSA can be readily purified from bovine blood[2].
BSA is a small, stable and moderately non-reactive protein. It has numerous biochemical applications, because of its ability to increase signal in assays, its lack of effect on many biochemical reactions and its low cost. It was often used as a blocker in immunohistochemistry[3], which is the process that uses antibodies to identify antigens in cells, and tissue sections are often incubated with BSA blockers to bind nonspecific binding sites[4]. The BSA blocker improves sensitivity by decreasing background noise as the sites are covered with the moderately non-reactive protein[5]. During this process, minimization of nonspecific binding of antibodies is essential in order to acquire the highest signal to noise ratio[6]. This binding of BSA to nonspecific binding sites increases the chance that the antibodies will bind only to the antigens[7]. BSA is also used as a nutrient in cell and microbial culture and is also commonly used to determine the quantity of other proteins[8], by comparing an unknown quantity of protein to known amounts of BSA.
Ferrocene (Fc) is an organometallic compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal Fe atom. The discovery of ferrocene and its many derivatives leads to the rapid growth of organometallic chemistry. And also, ferrocene and its derivatives have lots of applications, for examples, they can be used in asymmetric catalysis and enantioselective synthesis, and are widely used as redox sensors which can be incorporated into large proteins to act as redox relays[9]. Ferrocenes exhibit a well-characterized one-electron reversible oxidation wave and are useful as an electrochemical probe in the development of electrochemical sensor technologies that enable the detection of a wide range of biomolecules[10], especially for ferrocene dicarboxylic acid, i.e. Fc(COOH)2. Our previous work studied the interaction of ferrocene derivatives and heme to expand their potential applications[11].
Herein, we take advantage of Fc(COOH)2 as yellow compounds, and BSA shows optical property, and they all have some functional groups which can be used to study their interaction by UV-Vis, it can provide a new way to explore the quantitative detection of BSA based on the reaction of Fc(COOH)2 and BSA.
A UV-5300 spectrophotometer (Japan’ s Hitachi company) was used to perform all spectral experiments. All reagents used were of analytical grade. Fc(COOH)2 and BSA were purchased from Shanghai Macklin Biochemical Co., Ltd.(Shanghai, China). All aqueous solutions were prepared with Milli-Q water.
0.5 mmol· L-1 stock solution of Fc(COOH)2 was prepared by dissolving Fc(COOH)2 in 10.0 mmol· L-1 NaOH solution (pH=8.0). 2.0 g· L-1 stock solution of BSA was prepared by dissolving BSA in water. All working solutions were prepared by diluting the stock solution.
(1) UV-Vis spectra of Fc(COOH)2, BSA and their interaction
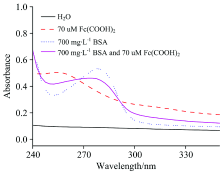
Because Fc(COOH)2 belongs to Lewis acid, it can dissolve well in weak basic solution, so in experimental section, stock solution of Fc(COOH)2 was prepared by dissolving Fc(COOH)2 in diluted NaOH solution (pH=8.0), and BSA are soluble in aqueous solution which was prepared by Milli-Q water, and the UV-Vis method can be used for the studies of their interaction which can provide a new way to explore the quantitative detection of BSA. Fig.1 shows UV-Vis spectra of Fc(COOH)2 and its linear relationship of absorbance and concentration in range of 10~190 μ mol· L-1, Fig.2 shows UV-Vis spectra of BSA and its linear relationship of absorbance and concentration in range of 100~1 900 mg· L-1, Fig.3 shows the UV-Vis spectra of 70 μ mol· L-1 Fc(COOH)2, 700 mg· L-1 BSA and their interaction (taken H2O as baseline).
It can be seen that the λ max of Fc(COOH)2 is 255 nm, and the linear equation of absorbance and concentration is Y=0.112 9+0.005 2X(r=0.998 4) in the range of 10~190 μ mol· L-1, and the λ max of BSA is 277.5 nm, and the linearity equation of the absorbance and concentration is Y=0.000 5+0.115 2X (r=0.999 2) in the concentration range of 100~1 900 mg· L-1 in Fig.1 and Fig.2. When 700 mg· L-1 BSA was mixed with 70 μ mol· L-1 Fc(COOH)2, λ max shifted to 275 nm to show the existed reaction (Fig.3). From the review of absorbance, before the reaction, the absorbance of Fc(COOH)2 and BSA are 0.504 and 0.533, respectively. After the reaction, the absorbance of new broad peak is about 0.458 confirming the existing reaction.
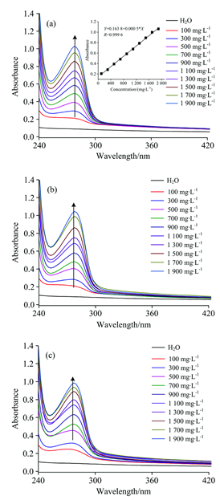 | Fig.4 UV-Vis spectra of 70 μ mol· L-1 Fc(COOH)2 and different concentration of BSA reaction for 0.1 h (a), 24 h (b) and 96 h (c) |
(2) Fixed concentration of Fc(COOH)2
In order to figure out the mechanism interaction between Fc(COOH)2 and BSA, a titration experiment was carried out, that is to fix the concentration of Fc(COOH)2 as 70 μ mol· L-1, reacting with different concentration of BSA (100~1 900 mg· L-1). Fig.4 shows UV-Vis spectra of 70 μ mol· L-1 Fc(COOH)2 and different concentration of BSA reaction for 0.1, 24, 96 h, respectively.
From Fig.4, it can be seen that when the concentration of Fc(COOH)2 is fixed, not only the absorbance of BSA increases with the increase of BSA concentration, but also the absorbance of Fc(COOH)2 increases with the increase of BSAconcentration. When the reaction time increases from 0.1 h to 24 and 96 h, the absorbance at maximum absorption wavelength (λ max=275 nm) has a little change, taking 1 900 mg· L-1 BSA as example, the absorbance change from 1.062 to 1.045 and 0.986, after 24 and 96 h reaction, respectively. So it can be concluded that the whole solution is unstable to show that there exists interaction between Fc(COOH)2 and BSA.
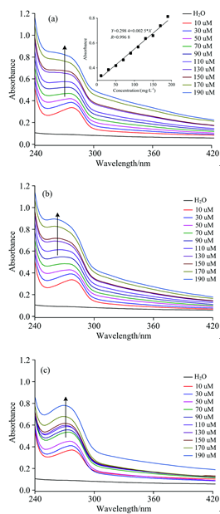 | Fig.5 UV-Vis spectra of 700 mg· L-1 BSA and different concentration of Fc(COOH)2 reaction for 0.1 h (a), 24 h (b) and 96 h (c) |
(3) Fixed concentration of BSA
Now the concentration of BSA is fixed as 700 mg· L-1, reacting with different concentration of Fc(COOH)2 (10~190 μ mol· L-1). Fig.5 shows UV-Vis spectra of 700 μ mol· L-1 BSA and different concentration of Fc(COOH)2 reaction for 0.1, 24, 96 h, respectively.
From Fig.5, it can be seen that when the concentration of BSA is fixed, not only the absorbance of Fc(COOH)2 increases with the increase of Fc(COOH)2 concentration, but also the absorbance of BSA increases with the increase of Fc(COOH)2 concentration. When the reaction time increases from 0.1 h to 24 and 96 h, the absorbance at maximum absorption wavelength (λ max=275 nm) has a little change, taking 190 μ mol· L-1 Fc(COOH)2 as example, the absorbance change from 0.813 to 0.794 and 0.750 after 24 h and 96 h reaction, respectively. So it can also be concluded that the whole solution is unstable to show that there exists interaction between Fc(COOH)2 and BSA as shown in results of fixed Fc(COOH)2 concentration.
From Fig.3, Fc(COOH)2 and BSA show their λ max at 255 and 277.5 nm, respectively. After the reaction, it shows λ max at 275 nm. After the reaction of Fc(COOH)2 and BSA, the UV-Vis spectra is different from UV-Vis spectra of Fc(COOH)2, and BSA. From the review of absorbance, before the reaction, the absorbance of Fc(COOH)2 and BSA are 0.504 and 0.533, respectively. After the reaction, the absorbance of new broad peak is about 0.458 confirming there existed reaction. From results of fixed concentration of Fc(COOH)2 and BSA, although one stuff concentration is fixed, its absorbance increases with increase of another stuff concentration, when considering that Fc(COOH)2 and BSA have free -COOH group, so the hydrogen bond interactions occurring between Fc(COOH)2 and BSA to form intermolecular hydrogen bond[12, 17], lead to the growth of molecular chain, since the bigger molecular can absorb more energy and increase the absorbance. Meanwhile, bigger molecules are more unstable, so the absorbance decreases with the increase of reaction time.
In this work, the interactions between Fc(COOH)2 and BSA was studied by UV-Vis spectroscopy. The results showed that the interaction occurr between Fc(COOH)2 and BSA to form intermolecular hydrogen bond based on — COOH/— NH— and — COOH groups. It can provide a new way to explore the quantitative detection of BSA based on the reaction of Fc(COOH)2 and BSA.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|