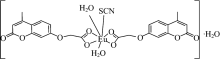
A new europium (Ⅲ) complex containing (4-Methyl-2-oxo-2H-chromen-7-yloxy)-acetic acid moiety (CMMC) was synthesized, characterized, and confirmed as antitumor agent and fluorescent probe. The spectroscopic measurements of Eu(Ⅲ) in the presence of CMMC were obtained in different solvents. The results show that the strongest Eu(Ⅲ) emission bands were monitored in iso-propyl alcohol while the weakest Eu(Ⅲ) emission band was observed in acetonitrile. The interaction of Eu(Ⅲ)-(CMMC)2 complex with DNA was monitored using absorption and emission techniques. From fluorescence titration measurements, the binding constants of DNA with Eu(Ⅲ)-(CMMC)2 complex were found to be 1.04×105 L·mol-1 in Tris-HCl and 1.17×107 L·mol-1 in DMSO-Tris-HCl buffer (9∶1 V/V). Hypochromism was observed from the absorption titration experiment which indicates the intercalation of Eu(Ⅲ)-complex between the base pair of DNA. This result further confirmed by fluorescent Ethidium bromide displacement assay. The fluorescence calibration curve was used for the determination of DNA with LOD of 1.2 ng in DMSO-Tris-HCl buffer (9∶1 V/V) and 5 ng in Tris-HCl buffer. The preliminary antitumor investigation shows promising cytotoxicity against MDA-MB-231, MCF-7 (mammary cancer), and PC-3 (prostate carcinoma) cell lines with IC50 values of 40.63, 25.42 and 30.25 μmol·L-1, respectively.
Extensive efforts and researches were employed to treat cancer where it is the second deadly disease in the world. New synthetic drugs were created based on their interaction with the nucleic acid to kill the cancer cell in vitro and in vivo cultures[1, 2, 3, 4]. The chemotherapeutic treatments of cancer have the challenge to find the new anticancer compounds with minimal side effects.
Recently, The coordination complex has been employed as antitumor chemotherapeutic agent and sensing nucleic acids[3, 4]. The lanthanide (Ⅲ ) ions have strong binding with biological molecules, low toxicity, long luminescence life time, and large stocks shift. The lanthanide ions should be including an organic chromophore (an antenna) to exhibit significant fluorescence intensity. Therefore, they can be used as fluorescent probe[5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19] in many fields, such as environmental, chemical, and biological applications[20, 21, 22, 23].They are used as fluorescence imaging agents[24], sensors[25, 26] and as fluorescent probes to study nucleic acids[27].
Coumarin derivatives and their metal complexes have many pharmaceutical functions[28, 29, 30, 31, 32] such as radical scavenging properties[33, 34], anti-oxidants[35, 36], enzyme inhibitors[37, 38], anti-viral[39], anti-tumor activity[26], and in the field of optoelectronic materials[40, 41, 42, 43] as well. The carboxylic group of CMMC (Fig.1) can coordinate to Europium (Ⅲ ) metal ions leading to enhance the biological activity and fluorescence intensity.
In the present work, the absorption and fluorescence properties of CMMC will be monitored at different solvents. A novel Eu(Ⅲ )(CMMC)2 complex was obtained from the reaction of Eu(Ⅲ ) metal ion with the CMMC ligand in the presence of potassium thiocyanate. The thermal analysis, IR spectroscopy, and elemental analysis will be used to characterize the newly synthesized Eu(Ⅲ )-complex. The interaction mode of DNA with Eu(Ⅲ )-complex has been investigated using spectroscopic methods. The fluorescence titration method should be useful for the detection of DNA. The antitumor activity of Eu(Ⅲ )-(CMMC)2 complex has been screened against MB-231, MCF-7 (mammary cancer), and PC-3 (prostate carcinoma) cell lines using colorimetric MTT assay.
EuCl3· 6H2O, Ethidium bromide (EB), potassium thiocyante, and calf thymus nucleic acid (ct-DNA) were purchased from Sigma Chemical Co. and were used without purification. The purity and concentration of ct-DNA in 0.1 mol· L-1 aqueous Tris-HCl buffers (pH 7.2) were checked by absorption measurements as described in literature [44].
CMMC ligand and its Eu(Ⅲ )-complex were synthesized according to the literature[45, 46]. A solution of potassium thiocyanate (10.8 mmol) in ethanol was added to EuCl3· 6H2O (1.7 mmol) in ethanol. The potassium chloride was precipitated and was removed by filtration. A solution of CMMC ligand (5.40 mmol) in ethanol was added to the filtrate with stirring. The obtained complex was collected, purified, and characterized by filteration, washing with hot ethanol, and instrumental analyses.
Elementar vario, Bruker Alpha with KBr discs, and a Shimazdu TG-DTG were used to elemental, IR, and thermal analysis, respectively. A Perkin-elmer lambda 20 UV-Vis and a Jasco 6300 spectrophotometer were included to absorption and fluorescence measurements. pH measurements were carried out on (pH-220 L) pH- meter.
Fluorescence and absoption titration measurements were performed by adding different concentrations of ct-DNA (0.1~50 μ mol· L-1) to Eu(Ⅲ )-CMMC complex ( 20 μ mol· L-1) in 0.1 mol· L-1 Tris-HCl buffer or in DMSO-Tris-HCl (9∶ 1, V/V). In fluorescent ethidium bromide displacement assay, EB solution was added to DNA solution. After incubation of EB solution with DNA solution for suitable time (3 h), a series Eu(Ⅲ )-(CMMC)2 complex concentrations were added to the EB-DNA mixture. Fluorescence measurements were monitored between 520 and 700 nm with excitation wavelength of 510 nm. The europium metal ion concentration in synthesized complex was determined complexometrically as reported in literature[47].
Human tumor cell lines including MCF-7, MDA-MB-231 (breast cancer), and PC-3 (prostate cancer) were obtained from the National Cancer Institute (NCI, Cairo, Egypt). They were cultured and antiproliferative activities were evaluated by using colorimetric MTT (3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide) method assay as previously reported[48]. The effect of 0.5% DMSO used in each assay on the growth inhibition activity of cell lines was also evaluated.
The molecular docking studies were done using Molecular Operating Environment (MOE) program[49] to investigate the binding mode and intermolecular interaction of Eu(Ⅲ )-(CMMC)2 complex with G-quadruplex DNA (PDB ID, 3QSF). The ligand was drawn in the MOE 2014.8. The geometry was optimized through molecular dynamic method with a gradient cut off value of 0.05 Kcal· mol-1· Å -1. The crystal structure of the active sites was obtained from the protein data bank. Molecular system optimization involves preparing the structure to be docked and the water molecules and co-crystallized ligands were removed.
Table 1 listed the analytical data for the synthesized Eu(Ⅲ )-(CMMC)2 complex and its parent ligand CMMC. The molecular formula of the synthesized compound may be [Eu(Ⅲ )(CMMC)2(SCN)(H2O)2]. H2O (Fig.2) as predicted from the elemental analytical data.
| Table 1 Elemental analytical data for the Eu(Ⅲ )-(CMMC)2 complex |
In the IR spectrum of the CMMC ligand, the highly intensity absorption bands at 1 733 and 1 616 cm-1 were attributed to the carbonyl and C≡ C group, respectively. While the broad band at 3 426 cm-1 is assigned to the OH group. The IR spectrum of Eu(Ⅲ )-complex (Fig.3) exhibited short (2 059 cm-1) wiich is assigned to the thiocyanate (NCS)[50]. The weak intensity band of Eu-O mode was observed at 454 cm-1. The carbonyl group in complex downshifted to 1 702 cm-1 due to the coordination with Eu(Ⅲ ) ion.
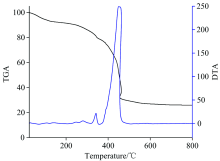
The thermogravimetric (TG) and differential thermal gravimetry (DTG) experiments (Fig.4) of the Eu(Ⅲ )-(CMMC)2 complex were performed to confirm its chemical composition through the steps of weight losses. The loss of non coordinating water molecule (H2O; calculated=2.46%; TG=2.01%) was detected as the first mass loss. This step was accompanied by an endothermic effect (123 ℃) on the DTA curve. The peaks were observed at 280 and 249 ℃ with endothermic peaks indicating the removal of thiocyanate group and coordinating water molecules (2H2O), respectively. The decomposition of CMMC ligand (calculated: 49.04%; TG=49.19%) with significant weight loss of 49.18% was found from 363 to 505 ℃. The decomposition of the CMMC ligand was reflected by endothermic effect at 450.21℃. The weight loss of 12.95% corresponding to the decarboxylation of CMMC was monitored at 362 ℃ (calculated: 12.05%; TG=12.95%). The residue is attributed to Eu2O3 (24.1%). The previous data confirmed the purposed chemical structure of synthesized complex (Fig.2).
2.2.1 UV-Vis absorption properties
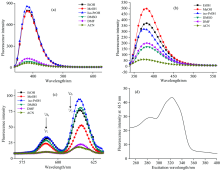
The absorption spectra of CMMC and Eu(Ⅲ )-(CMMC)2 complex were recorded in different solvents as shown in Fig.5 and the absorption properties of the studied compounds were listed in Table 2. The CMMC spectra show an absorption around 320 nm attributed to π — π * transition while the absorption spectra of Eu(Ⅲ )-(CMMC)2 complex exhibited changing in the absorption intensity with hypsochromic shift. The most absorption enhancement of Eu(Ⅲ )-complex was observed in methanol and iso-propanol and the most blue shift was monitored in acetonitrile (AcN).
 | Fig.5 Absorption spectra of 2× 10-5 mol· L-1 CMMC (a) and 2× 10-5 mol· L-1 Eu(Ⅲ )-(CMMC)2 complex (b) in different solvents at room temperature |
| Table 2 The absorption properties of 2× 10-5 mol· L-1 CMMC ligand and Eu(Ⅲ )-CMMC (1∶ 1) binary system in different solvents at room temperature |
2.2.2 Steady-state fluorescence spectroscopy
To select the best excitation wavelength, the excitation fluorescence spectrum of Eu(Ⅲ )-CMMC complex at 616 nm was performed with the excitation range from 250 to 400 nm. From this experiment, the maximum absorption wavelength was selected as the most suitable excitation wavelength for further measurements [Fig.6(d)]. The fluorescence spectra of the CMMC and Eu(Ⅲ )-(CMMC)2 complex have been studied in different solvents. Figs.6(a)— (c) and Table 3 represented the fluorescence intensities at characteristic emission wavelengths. The fluorescence spectra of CMMC ligand show the maximum emission band at about 383 nm. The emission band of Eu(Ⅲ )-(CMMC)2 complex centered at 615 nm (5D0→ 7F2) is higher than that at 591 nm (5D0→ 7F1), indicating that the Eu(Ⅲ ) ion is in a center of symmetric coordination site[51]. The addition of Eu(Ⅲ ) ions enhances the fluorescence intensity of ligand in DMSO and DMF. The fluorescence spectra show the strong blue shift in iso-propyl alcohol and acetonitrile. The poorest intramolecular energy transfer was observed in DMF and ACN while the strongest energy transfer was observed in ethanol, DMSO, and methanol.
| Table 3 Emission peak positions and relative fluorescence intensity of CMMC ligand and its complex Eu(Ⅲ )-(CMMC)3 in different solvents |
2.3.1 UV-Vis absorption
The interaction mode of DNA with the Eu(Ⅲ )-(CMMC)2 complex through groove, electrostatic, and intercalative binding can be identified by absorption spectroscopy[52, 53]. Fig.7 showed clear hypochromicity in the absorption spectra of the CMMC and Eu(Ⅲ )-complex in the presence of DNA. The hypochromicity in π — π * transition indicated the intercalative binding of CMMC and the binary Eu(Ⅲ )-CMMC complex with the base pairs of DNA[54]. The intercalative mode is the strongest binding modes[52].
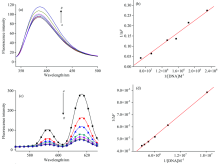
The fluorescence intensity of Eu(Ⅲ )-complex and CMMC ligand in the presence of different concentration of DNA exhibited a pronounced change in Tris-HCl buffer and DMSO-Tris (9∶ 1, V/V) as shown in Figs.8 and 9. It cannot monitor the interaction of Eu(Ⅲ ) complex with DNA at the characteristic emission band of Eu(Ⅲ ) in Tris-HCl buffer due to the fact that it was completely quenched under experimental condition. Therefore, the fluorescence intensity of Eu(Ⅲ )-complex in the presence of DNA was monitored at 385 nm corresponding to CMMC in Eu-complex. DNA remarkably enhanced the fluorescence intensity of Eu(Ⅲ )-CMMC complex with slightly red shift from 385 to 388 nm [Fig.8(a)]. On the other hand, the addition of DNA to CMMC caused obvious reduction in emission intensities without change in the maximum emission.
The characteristic emission band for Eu(Ⅲ ) at 616 nm in Eu(Ⅲ )-CMMC was observed in DMSO-Tris (9∶ 1 V/V). Therefore, the interactions of DNA dissolved in DMSO-Tris (9∶ 1 V/V) with Eu(Ⅲ )-CMMC complex and CMMC free ligand were also investigated (Fig.9).
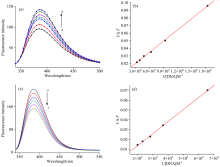
The fluorescence titration measurements of DNA with Eu(Ⅲ )-(CMMC)2 complex (Figs.8 & 9) were employed to determine the stoichiometry and binding constant as follows[55]
where Δ F is the fluorescence intensity difference in the absence (F0) and presence of DNA (F), respectively. Fl is the limiting intensity of fluorescence and α is 1/F0(F0-Fl). A plot of 1/Δ F versus 1/[DNA] according to Eq. (1) should give a straight line (r=0.999) with stoichiometry of the formed complex 1∶ 1. K denotes the binding constant of Eu(Ⅲ )-(CMMC)2 with DNA and was listed in Table 4. The data showed that the binding ability of Eu(Ⅲ )-complex with DNA is easier and stronger than that of CMMC ligand. There is no considerable difference between the association constant of the free ligand with DNA in Tris-HCl or in DMSO-Tris. However, there is a great difference between the two media on the association constant of the Eu(Ⅲ )-CMMC complex with DNA. This could be assigned to the intercalation of the predominant DMSO molecule in the medium with Eu(Ⅲ )-CMMC forming ternary complex Eu(Ⅲ )-CMMC-DMSO which acquire more intercalating effect with the base pairs of DNA.
| Table 4 Binding parameters of Eu(Ⅲ )-(CMMC) and CMMC free ligand with DNA according to Eq.1 at pH 7.4 |
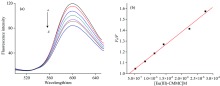
The fluorescent EB displacement assay was further monitored to indentify the binding mode of DNA-Eu(Ⅲ )-complex. EB strongly intercalates between DNA base pairs. The fluorescence quenching measurements of EB-DNA system by the addition of a third molecule[56] can be analyzed to evaluate the binding ability of Eu(Ⅲ )-CMMC complex to ct-DNA. Fig.10 showed fluorescence quenching of the EB-DNA system in the presence of Eu(Ⅲ )-(CMM)2 complex, indicating the displacement of EB from the EB-DNA system by Eu(Ⅲ )-(CMMC)2 complex[57, 58].
The Stern-Volmer quenching equation can be used to analyze the displacement fluorescence assay: F0/F=1+KSV[Q], where Q is the quencher concentration. KSV is a Stern-Volmer constant. F and F0 represent the fluorescence intensities in the presence and absence of the complex, respectively. From KSV value for complex (2.29± 0.5)× 105 L· mol-1, the latter could intercalate DNA and squeeze EB from DNA double helix[57]. The binding constant was determined from C50 values as follows
where C50 value is the concentration of the complex reducing the luminescence of the EB-DNA system by 50%. KEB is the binding constant of EB to DNA (KEB=1× 107 L· mol-1) and is typical for intercalator into DNA[59].
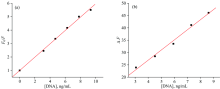
The luminescence titration experiments of the Eu(Ⅲ )-(CMMC)2 complex in the present different concentrations of DNA can be considered as calibration graphs for the DNA determination (Fig.11). The good linear ranges were 2~50 ng for DNA with the correlation coefficients of 0.997. The detection limits were 1.2 ng for DNA in DMSO-Tris (9∶ 1 V/V) and 5 ng for DNA in 0.1 mol· L-1 Tris-buffer.
The interference of foreign substances with the DNA was examined to testify the selectivity of the proposed method. Table 5 gives the tolerance value of the coexisting substances obtained from the changes of fluorescence intensity. Some metal ions, such as N
| Table 5 Tolerable concentration of coexisting substances in the Eu(Ⅲ )-CMMC complex (10 μ mol· L-1) with DNA (10.0 μ mol· L-1) |
| Table 6 Common luminescence probes for nucleic acid determination |
In vitro, the synthesized Eu(Ⅲ )-(CMMC)2 complex and the well known anticancer drug carboplatin have been tested for their cytotoxic activities against three cancer cell lines of human origin including MCF-7, MDA-MB-231(mammary cancer), and PC-3 (prostate carcinoma cell line) by applying the MTT colorimetric assay. The Eu(Ⅲ )-(CMMC)2 complex showed activity against MCF-7, MDA-MB-231, and PC-3 cells with IC50 values at 40.63, 25.42 and 30.25 μ mol· L-1, respectively. It is more potent against MDA-MB-231 cell lines than against MCF-7 and PC-3 cell lines, suggesting that the designed compounds possess high selectivity for MDA-MB-231 cancer cell lines.
The molecular docking studies were done using MOE program to investigate the binding strength of Eu(Ⅲ )-(CMMC)2 complex with DNA, as well as the intermolecular interaction. Figure 12 shows the presence of arene-arene interactions (π — π staking) between the Eu(Ⅲ )-(CMMC)2 complex and guanine base of DNA (DG1008) with interaction distance of 3.55 Å and a hydrogen bond between DT1009 and coumarin with bonding length 3.35 Å . The binding involves hydrophobic interactions between the non-polar parts of the G-quadruplex nucleotides and coumarin moiety. The obtained conformations were ranked based on the lowest free binding energy. Accordingly, the most stable complex formed between the DNA and Eu(Ⅲ )-(CMMC)2 complex [Fig.12(a)] was found to possess -7.05 kcal· mol-1 binding energy which indicates that the Eu(Ⅲ )-(CMMC)2 complex has a good binding affinity to DNA. Docking result is in well agreement with the above spectral assays.
A new fluorescent probe Eu(Ⅲ )-(CMMC)2 complex for sensing DNA was synthesized and characterized by spectroscopic methods. In this paper, fluorescence and UV-Vis absorption spectra were used to confirm the interaction between CMMC ligand and Eu(Ⅲ ) ion in different solvents. Also, the spectroscopic methods were used to examine the interaction of CMMC free ligand and its binary complex Eu(Ⅲ )-CMMC with DNA in Tris-HCl buffer and DMSO-Tris (9∶ 1 V/V).The binding constant of Eu(Ⅲ )-CMMC complex in DMSO-Tris is greater than that in Tris-buffer. From the fluorescence titration experiments, the low detection limits of DNA were found to be 1.2 ng in DMSO-Tris (9∶ 1 V/V) and 5 ng in Tris-HCl buffer. Hypochromism was observed from the absorption experiment which indicates the intercalation between Eu(Ⅲ )-complex and base pair of DNA. The interacting mode was further confirmed by fluorescent ethidium bromide displacement assay and molecular docking.
We are grateful to the Head, Department of Chemistry, Faculty of Science, Suez Canal University, Ismailia, Egypt for extending laboratory facilities.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|